|
 |
| Korean J Anesthesiol > Volume 77(2); 2024 > Article |
|
Abstract
Background
Hyperglycemia has shown a negative association with cognitive dysfunction. We analyzed patients with high preoperative blood glucose level and hemoglobin A1c (HbA1c) level to determine the prevalence of postoperative delirium.
Methods
We reviewed a database of 23,532 patients with diabetes who underwent non-cardiac surgery. Acute hyperglycemia was defined as fasting blood glucose > 140 mg/dl or random glucose > 180 mg/dl within 24 h before surgery. Chronic hyperglycemia was defined as HbA1c level above 6.5% within three months before surgery. The incidence of delirium was compared according to the presence of acute and chronic hyperglycemia.
Results
Of the 23,532 diabetic patients, 21,585 had available preoperative blood glucose level within 24 h before surgery, and 18,452 patients reported levels indicating acute hyperglycemia. Of the 8,927 patients with available HbA1c level within three months before surgery, 5,522 had levels indicating chronic hyperglycemia. After adjustment with inverse probability weighting, acute hyperglycemia was related to higher incidence of delirium (hazard ratio: 1.33, 95% CI [1.10,1.62], P = 0.004 for delirium) compared with controls without acute hyperglycemia. On the other hand, chronic hyperglycemia did not correlate with postoperative delirium.
Conclusions
Preoperative acute hyperglycemia was associated with postoperative delirium, whereas chronic hyperglycemia was not significantly associated with postoperative delirium. Irrespective of chronic hyperglycemia, acute glycemic control in surgical patients could be crucial for preventing postoperative delirium.
Delirium is a common complication following surgery and is described as a sudden change in mental state leading to fluctuations in consciousness or cognitive function [1]. Postoperative delirium, although typically temporary, has been linked to increased hospital stay, higher medical expenses, increased risk of complications, higher readmission rates, and even higher in-hospital mortality [2–5]. The development of postoperative delirium is thought to be due to a heightened susceptibility of the brain to external stressors, but the specific cause is not well understood. The interplay between risk factors and triggers makes it challenging to establish a protocol for preventing delirium.
Diabetes, a well-established risk factor for postoperative complications, is also linked to cognitive dysfunction, that could be caused by poor glycemic control [6]. Specifically, hyperglycemia is common in surgical patients, with a prevalence of 20% to 40%, and many of those patients have diabetes [7,8]. Increasing research suggests a correlation between perioperative hyperglycemia and negative clinical outcomes in surgical patients [8,9]. Theoretically, perioperative hyperglycemia leads to oxidative stress that can weaken the blood–brain barrier and result in neuronal inflammation [10]. The relationship between hyperglycemia and delirium has been shown in patients who are hospitalized, patients undergoing surgery [11,12], and those with poorly controlled diabetes [13]. Despite the need, a guideline for managing blood glucose levels in diabetic patients during surgery has not yet been established. In this study, we investigated the relation delirium and high blood glucose levels by conducting two separate analyses for acute and chronic hyperglycemia in diabetic patients undergoing non-cardiac surgery. Our results could aid in the development of future guidelines for managing blood glucose levels in the perioperative period.
The Institutional Review Board of Samsung Medical Center approved this study (SMC 2021-06-078) and waived the need for written informed consent from participants due to the nature of the study as explained below. This research was conducted in accordance with the Declaration of Helsinki, 2013 and results are reported according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
We conducted the analysis using the data from Non-cardiac Operation registry (NoCop) database for our institution. The NoCop registry is a single-center registry with de-identified data for 203,787 consecutive adult patients who underwent non-cardiac surgery, between January 2011 and June 2019. Data were extracted automatically using Clinical Data Warehouse that contains electronic hospital records for more than four million patients, including more than 900 million laboratory findings and 200 million prescriptions. The system can also pull data on mortalities from the National Population Registry of the Korea National Statistical Office using unique personal identification numbers.
Investigators independent of our study and blinded to the results used preoperative evaluation sheets and operation records to organize information about relevant variables. We also used International Classification of Diseases-10 codes to organize preoperative diagnoses. Non-diabetic patients and patients with preoperative diagnoses of delirium or dementia were excluded from this study. We divided our study population into groups and conducted two separate analyses after stratifying patients based on the presence of acute and chronic hyperglycemia. The acute hyperglycemia analyses considered patients with a blood glucose level measured within 24 h before surgery, and the chronic hyperglycemia analyses considered patients with a hemoglobin A1c (HbA1c) level measured within three months before surgery.
Acute hyperglycemia was defined as at least one fasting blood glucose level above 140 mg/dl (7.77 mmol/L) or a random blood glucose level above 180 mg/dl (9.99 mmol/L) within 24 h before surgical incision. These levels were set according to the guidelines recommended by the American Diabetes Association and the American Association of Clinical Endocrinology [14]. Chronic hyperglycemia was defined as HbA1c level above 6.5% within three months before surgery [15]. Following institutional protocol, glucose concentrations were selectively measured during preoperative evaluation for patients with extensive medical histories or undergoing high-risk surgeries. Surgical risk was stratified according to the European Society of Cardiology/European Society of Anesthesiology guidelines on non-cardiac surgery [16]. According to these guidelines, surgeries were categorized into low risk, intermediate risk, and high risk based on the probability of cardiovascular risk occurrence: less than 1% as low risk, 1%–5% as intermediate risk, and over 5% as high risk. Patient physical status was classified by each attending anesthesiologist according to the American Society of Anesthesiologists Physical Status Classification System [17].
The primary endpoint was postoperative delirium, diagnosed by a psychiatrist using the Confusion Assessment Method (CAM), within 30 days after surgery. During postoperative care, patients with acute confusion or behavioral change were examined by the department of neuropsychology at the discretion of the attending clinician. For secondary endpoints, we analyzed mortality during one and three years of follow up.
For baseline characteristics, continuous variables are presented as means ± standard deviation (SD) or medians with interquartile ranges and were compared using a t-test or Mann-Whitney test, as appropriate. Categorical variables are reported as numbers and percentages and were compared using the Chi-square or Fisher’s exact test, as appropriate. Statistical significance was set at P < 0.05. We used inverse probability weighting (IPW) to balance the distribution of all available covariates between the groups while preserving the total number of patients. Specifically, we considered all available potential confounding factors in our analysis to evaluate the impact of hyperglycemia on postoperative delirium such as baseline characteristics (gender, age, and body mass index), and previous medical history such as hypertension, chronic kidney disease, cardiovascular conditions, and chronic obstructive pulmonary disease. We also retained blood test results, electrolyte levels, intraoperative event, and surgical risk. Stabilized weights of these variables inversely proportional to the marginal probability of hyperglycemia were used [18]. Through this method, the weights assigned to patients with higher values were the inverse of the propensity score, while patients with lower values received corresponding weights. The standardized mean difference was calculated to assess the balance of the covariates, and an absolute standardized difference value less than 10% was considered negligible. This diminished the confounding effect of relevant variables while estimating the effect of hyperglycemia on the outcomes. We then compared the risk of outcomes using a Cox proportional hazard regression model to compare the risk of outcomes between the two groups. The results were presented using hazard ratio (HRs) with 95% CIs. Kaplan-Meier curves were generated, and the log-rank test was used to compare the cumulative incidence of delirium. The log-rank test was employed to compare the curves between the hyperglycemia and non-hyperglycemia groups. All statistical analyses were performed using R 4.2.0 (http://www.R-project.org/), and the ‘ipw’ package was employed for IPW adjustment [19].
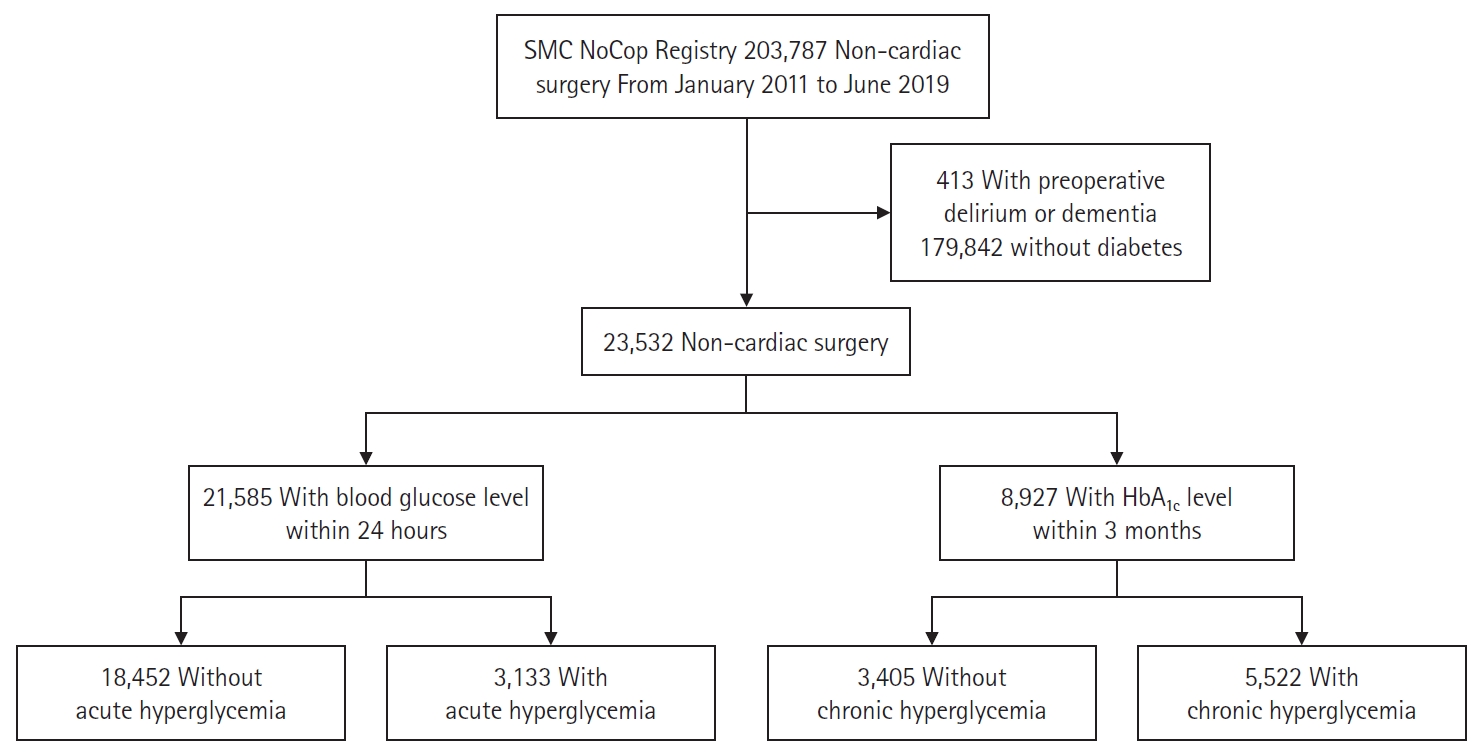
We excluded 413 patients with preoperative diagnoses of delirium or dementia and selected 23,532 (11.2% of the entire cohort) patients with diabetes. The baseline characteristics of patients with and without diabetes are presented in the supplemental material (Supplementary Table 1). The incidence of postoperative delirium in patients with diabetes (3.1%) was higher than that in patients without diabetes (1.2%). The flowchart of study patients is shown in Fig. 1.
In patients with diabetes, 21,585 (91.7%) had available blood glucose levels measured within 24 h before surgery. Acute hyperglycemia was observed in 3,133 (14.5%) of those patients and was more prevalent in males and in patients with more comorbidities than average. The baseline characteristics of patients with and without acute hyperglycemia are summarized in Table 1. Compared with the group without acute hyperglycemia, the acute hyperglycemia group showed a significantly higher risk of delirium (2.6% vs. 6.2%, HR: 2.43, 95% CI [2.06, 2.87], P < 0.001). The significance of the results persisted after adjustment with IPW (HR: 1.33, 95% CI [1.10, 1.62], P = 0.004) (Table 2).
For chronic hyperglycemia analyses, we selected the 8,927 (37.9%) diabetic patients with available HbA1c level within three months before surgery. Baseline characteristics according to the presence of chronic hyperglycemia are shown in Table 3. Chronic hyperglycemia was not associated with delirium (3.5% vs. 3.3%, HR: 0.92, 95% CI [0.73, 1.16], P = 0.5 for delirium). The results persisted after adjustment with IPW (HR: 0.94, 95% CI [0.74, 1.19], P = 0.6) (Table 4). The Kaplan-Meier curves for delirium according to acute and chronic hyperglycemia are presented in Figs. 2 and 3. Acute hyperglycemia showed a significantly higher risk of delirium regardless of the presence of chronic hyperglycemia (HR: 2.11, 95% CI [1.54, 2.90], P < 0.001 for those without chronic hyperglycemia; HR: 2.87, 95% CI [1.82, 4.52], P < 0.001 for those with chronic hyperglycemia).
We investigated the relationship between hyperglycemia and delirium in diabetic patients undergoing non-cardiac surgery using two distinct analyses for acute and chronic hyperglycemia. After selecting the diabetic patients for analysis, we compared the incidence of postoperative delirium according to the presence of diabetes, and delirium was more commonly found in diabetic patients. Among diabetic patients, acute hyperglycemia was significantly associated with postoperative delirium and one- and three-year mortality, whereas chronic hyperglycemia showed a significant association only with one- and three-year mortality.
Diabetes has long been reported as a risk factor for cognitive dysfunction. Particularly in surgical patients, the presence of diabetes has been shown to be associated with higher rates of postoperative delirium and hyperglycemia than in the general population [6,9,20]. Several other studies have focused on perioperative hyperglycemia and the risk of postoperative delirium [21–23]. Although some current guidelines recommend perioperative glycemic control [14,24,25], no treatment strategy has been optimized [7]. We conducted two distinct analyses for acute and chronic hyperglycemia and found that acute hyperglycemia was significantly associated with postoperative delirium, whereas chronic hyperglycemia was not.
The association between acute hyperglycemia and postoperative delirium can be well explained. The first connection is neuro-inflammation that can be induced by hyperglycemia. Overactivation of glucose metabolism in hyperglycemic patients increases the generation of reactive oxygen species [26,27], as well as glycolytic intermediates, and the secretion of pro-inflammatory cytokines [28,29]. An imbalance between free radical generation and elution causes oxidative stress to neuronal cells and increased inflammatory secretion. Second, hyperglycemia is related to blood–brain barrier destruction [10,26]. The blood-brain barrier is a selective diffusion barrier that maintains homeostasis in the central neural system and restricts penetration of neurotoxic molecules. In an animal study, hyperglycemia was related to disruption of this barrier due to microvascular abnormalities [30]. Defects in the microvasculature of the brain could result in impaired neuronal function and increased delirium.
Unlike acute hyperglycemia, chronic hyperglycemia did not show a significant association with postoperative delirium. The relationship between chronic hyperglycemia and delirium was controversial in previous studies [21,31,32]. Although the presence of diabetes was consistently shown to be a risk factor for postoperative delirium, preoperative HbA1c level showed a significant association with postoperative delirium in one study [21] but not in another [32]. Our finding that chronic hyperglycemia was not associated with delirium can be explained by an increased tolerance to hyperglycemia [23]. Long-term exposure to high blood glucose can induce cell conditioning, such as control of the overflow of blood glucose into cells by regulating the expression of the glucose transporter in cell membranes to reduce glucose toxicity. Therefore, patients with chronic hyperglycemia within a certain range might be less vulnerable than others to the acute stress induced by surgery. However, chronic hyperglycemia has been reported to be a risk factor for cognitive dysfunction in long-term follow-up [12,33]. Thus, the clinical implication of our study is not that chronic hyperglycemia can be neglected, but that acute glycemic control might be important even in patients with chronic hyperglycemia.
Our study has several limitations. First, selection bias and information bias cannot be excluded from any observational study. Because our study was conducted in a single center where most of the patients are Asian, the results of this study cannot be generalized. Furthermore, residual confounding factors from unmeasured variables such as severity of diabetes and medication use, and intraoperative blood glucose level may have affected our results despite rigorous statistical adjustments. Second, we diagnosed delirium using the CAM score that might have underestimated the incidence of postoperative delirium. While the CAM score is widely used and validated, it relies on specific criteria and may not capture all nuances of delirium. Alternative scales, such as the Intensive Care Delirium Screening Checklist (ICDSC), offer advantages by utilizing dichotomous variables and incorporating non-verbal language assessments. These features could potentially provide a more comprehensive assessment of delirium. Also, we could not assess the severity or character of delirium because we were conducting a retrospective study. Third, we investigated only the preoperative glucose level. So, the effect of stress hyperglycemia that is common during surgery could not be considered. Despite those limitations, our results might be helpful in establishing guidelines for blood glucose management and prevention of delirium in diabetic patients.
In conclusion, preoperative acute hyperglycemia was associated with increased postoperative delirium and one- and three-year mortality, but chronic hyperglycemia did not show a significant association with postoperative delirium. Our findings underscore the potential importance of prioritizing acute glucose control before non-cardiac surgery regardless of long-term glucose control. Nevertheless, it is crucial to recognize that comprehensive care should encompass the management of chronic hyperglycemia, alongside acute glycemic control in diabetic patients undergoing non-cardiac surgery.
NOTES
Supplementary Material
Supplementary Table 1.
Baseline Characteristics and Outcomes of Patients with and without Diabetes.
Fig. 2.
Kaplan-Meier curves of cumulative postoperative delirium during 30 days after surgery, seperated by presence of acute hyperglycemia.
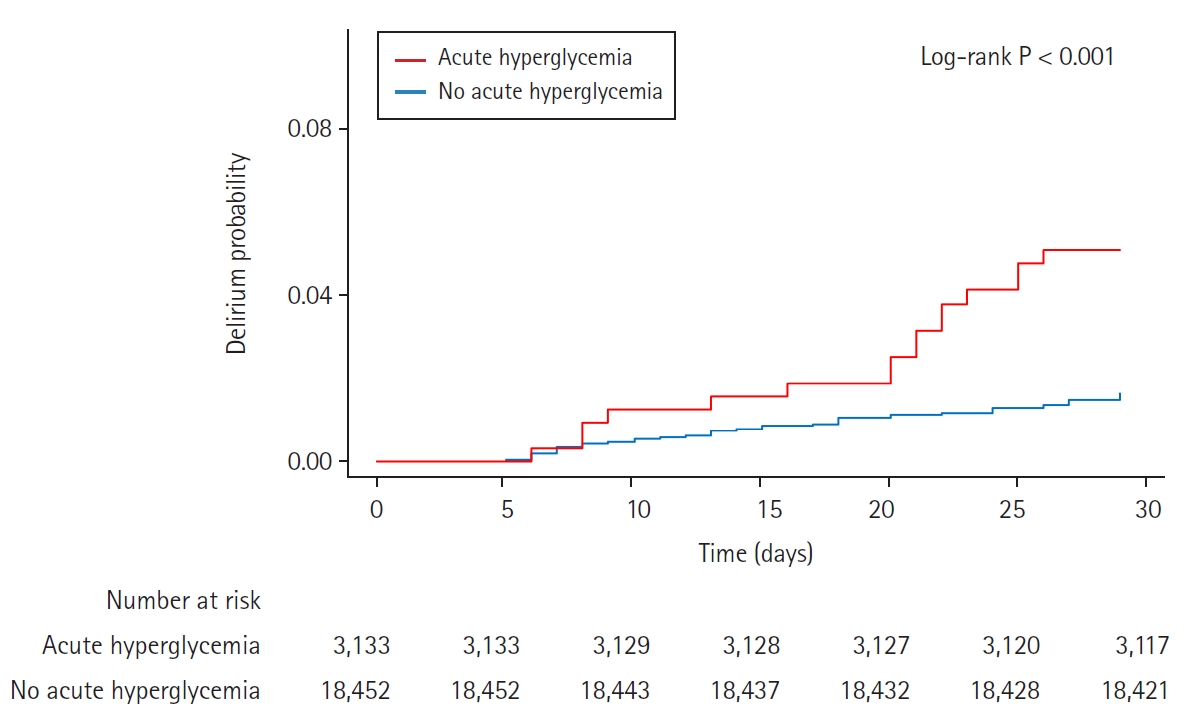
Fig. 3.
Kaplan-Meier curves of cumulative postoperative delirium during 30 days after surgery, seperated by presence of chronic hyperglycemia.
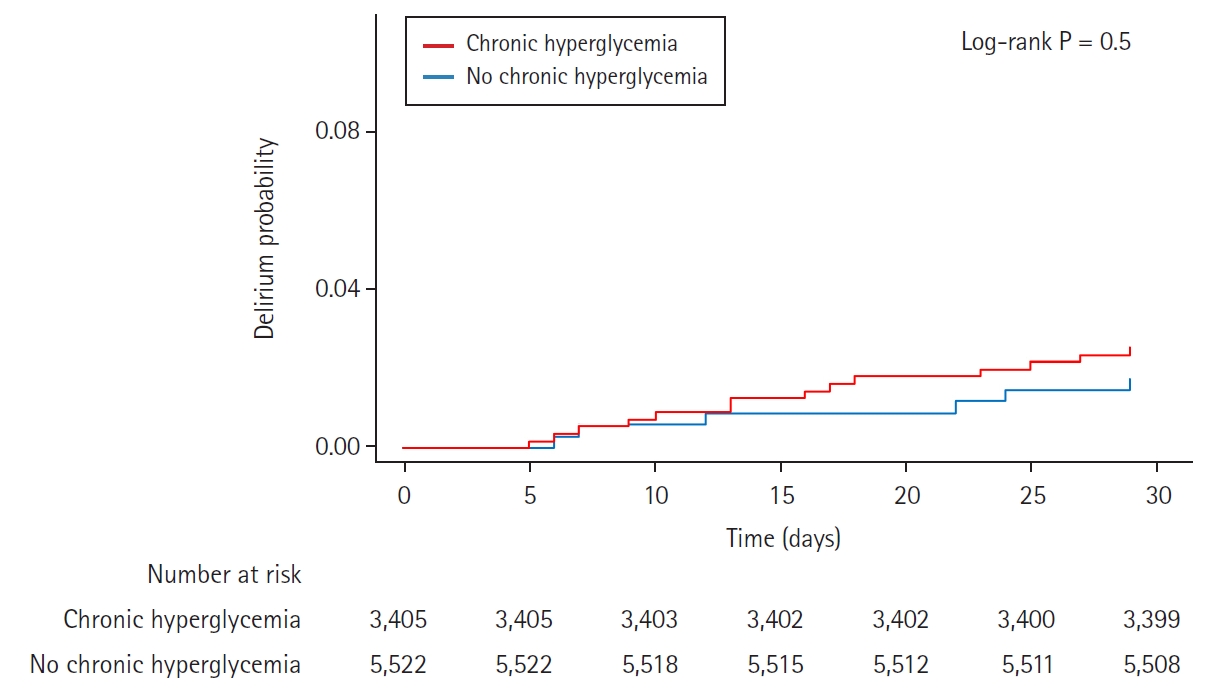
Table 1.
Baseline Characteristics according to Acute Hyperglycemia in Patients with Blood Sugar Test within 24 Hours before Surgery
Table 2.
Clinical Outcomes according to Acute Hyperglycemia
Table 3.
Baseline Characteristics according to Chronic Hyperglycemia in Patients with HbA1c within Three Months before Surgery
Values are presented as number (%) or mean ± SD. Surgical risk was stratified according to 2014 European Society of Cardiology/European Society of Anesthesiology guidelines. HbA1c : hemoglobin A1c, IPW: inverse probability weighting, ASD: absolute standardized difference. P value < 0.05 indicates statistical significance.
Table 4.
Clinical Outcomes according to Chronic Hyperglycemia
References
1. Swarbrick CJ, Partridge JS. Evidence-based strategies to reduce the incidence of postoperative delirium: a narrative review. Anaesthesia 2022; 77 Suppl 1: 92-101.



3. McCusker J, Cole MG, Dendukuri N, Belzile E. Does delirium increase hospital stay? J Am Geriatr Soc 2003; 51: 1539-46.


4. Schubert M, Schurch R, Boettger S, Garcia Nunez D, Schwarz U, Bettex D, et al. A hospital-wide evaluation of delirium prevalence and outcomes in acute care patients - a cohort study. BMC Health Serv Res 2018; 18: 550.




5. Wang YY, Yue JR, Xie DM, Carter P, Li QL, Gartaganis SL, et al. Effect of the tailored, family-involved hospital elder life program on postoperative delirium and function in older adults: a randomized clinical trial. JAMA Intern Med 2020; 180: 17-25.



6. Bordier L, Doucet J, Boudet J, Bauduceau B. Update on cognitive decline and dementia in elderly patients with diabetes. Diabetes Metab 2014; 40: 331-7.


7. Duggan EW, Carlson K, Umpierrez GE. Perioperative hyperglycemia management: an update. Anesthesiology 2017; 126: 547-60. Erratum in: Anesthesiology 2018; 129: 1053.



8. Frisch A, Chandra P, Smiley D, Peng L, Rizzo M, Gatcliffe C, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 2010; 33: 1783-8.




9. Kotagal M, Symons RG, Hirsch IB, Umpierrez GE, Dellinger EP, Farrokhi ET, et al. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg 2015; 261: 97-103.



10. Rom S, Zuluaga-Ramirez V, Gajghate S, Seliga A, Winfield M, Heldt NA, et al. Hyperglycemia-driven neuroinflammation compromises BBB leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol Neurobiol 2019; 56: 1883-96.




11. Windmann V, Spies C, Knaak C, Wollersheim T, Piper SK, Vorderwulbecke G, et al. Intraoperative hyperglycemia increases the incidence of postoperative delirium. Minerva Anestesiol 2019; 85: 1201-10.


12. Song Q, Dai M, Zhao Y, Lin T, Huang L, Yue J. Association between stress hyperglycemia ratio and delirium in older hospitalized patients: a cohort study. BMC Geriatr 2022; 22: 277.




13. Lopes R, Pereira BD. Delirium and psychotic symptoms associated with hyperglycemia in a patient with poorly controlled type 2 diabetes mellitus. Innov Clin Neurosci 2018; 15: 30-3.
14. Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009; 32: 1119-31.




15. Ko SH, Hur KY, Rhee SY, Kim NH, Moon MK, Park SO, et al. Antihyperglycemic agent therapy for adult patients with type 2 diabetes mellitus 2017: a position statement of the Korean Diabetes Association. Diabetes Metab J 2017; 41: 337-48.




16. Kristensen SD, Knuuti J, Saraste A, Anker S, Botker HE, Hert SD, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35: 2383-431.


17. Doyle DJ, Goyal A, Bansal P, Garmon EH. American Society of Anesthesiologists Classification. Treasure Island, StatPearls Publishing. 2021.

18. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34: 3661-79.




19. van der Wal WM, Geskus RB. ipw: an R package for inverse probability weighting. J Stat Softw 2011; 43: 1-23.

20. Karimian N, Niculiseanu P, Amar-Zifkin A, Carli F, Feldman LS. Association of elevated pre-operative hemoglobin A1c and post-operative complications in non-diabetic patients: a systematic review. World J Surg 2018; 42: 61-72.



21. Kotfis K, Szylinska A, Listewnik M, Brykczynski M, Ely EW, Rotter I. Diabetes and elevated preoperative HbA1c level as risk factors for postoperative delirium after cardiac surgery: an observational cohort study. Neuropsychiatr Dis Treat 2019; 15: 511-21.



22. Hermanides J, Qeva E, Preckel B, Bilotta F. Perioperative hyperglycemia and neurocognitive outcome after surgery: a systematic review. Minerva Anestesiol 2018; 84: 1178-88.


23. Marik PE, Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care 2013; 17: 305.



24. Akiboye F, Rayman G. Management of hyperglycemia and diabetes in orthopedic surgery. Curr Diab Rep 2017; 17: 13.




25. Jacobi J, Bircher N, Krinsley J, Agus M, Braithwaite SS, Deutschman C, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 2012; 40: 3251-76.


26. Bogush M, Heldt NA, Persidsky Y. Blood brain barrier injury in diabetes: unrecognized effects on brain and cognition. J Neuroimmune Pharmacol 2017; 12: 593-601.




27. Kumar P, Raman T, Swain MM, Mishra R, Pal A. Hyperglycemia-induced oxidative-nitrosative stress induces inflammation and neurodegeneration via augmented tuberous sclerosis complex-2 (TSC-2) activation in neuronal cells. Mol Neurobiol 2017; 54: 238-254.



28. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813-20.



29. Janelidze S, Hertze J, Nägga K, Nilsson K, Nilsson C, Wennström M, et al. Increased blood-brain barrier permeability is associated with dementia and diabetes but not amyloid pathology or APOE genotype. Neurobiol Aging 2017; 51: 104-12.



30. Sickmann HM, Waagepetersen HS. Effects of diabetes on brain metabolism--is brain glycogen a significant player? Metab Brain Dis 2015; 30: 335-43.



31. Rollins KE, Varadhan KK, Dhatariya K, Lobo DN. Systematic review of the impact of HbA1c on outcomes following surgery in patients with diabetes mellitus. Clin Nutr 2016; 35: 308-16.