 |
 |
| Korean J Anesthesiol > Volume 76(6); 2023 > Article |
|
Abstract
Background
Methods
Results
Conclusions
NOTES
Funding
This study was supported by Biomedical Research Institute Grant (20210033), Pusan National University Hospital.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Hyae Jin Kim (Conceptualization; Investigation; Writing – original draft; Writing – review & editing)
Soeun Jeon (Conceptualization; Data curation; Funding acquisition; Investigation; Visualization; Writing – original draft; Writing – review & editing)
Hyeon Jeong Lee (Conceptualization; Formal analysis; Methodology; Supervision; Writing – review & editing)
Jaeho Bae (Conceptualization; Investigation; Methodology; Supervision; Validation; Writing – review & editing)
Hyun-Su Ri (Formal analysis; Investigation; Writing – original draft)
Jeong-Min Hong (Investigation; Methodology; Visualization; Writing – review & editing)
Sung In Paek (Formal analysis; Investigation; Writing – original draft)
Seul Ki Kwon (Methodology; Visualization)
Jae-Rin Kim (Investigation; Methodology)
Seungbin Park (Investigation; Methodology; Validation)
Eun-Jung Yun (Investigation; Visualization)
Supplementary Material
Supplementary Fig. 1.
Fig. 1.
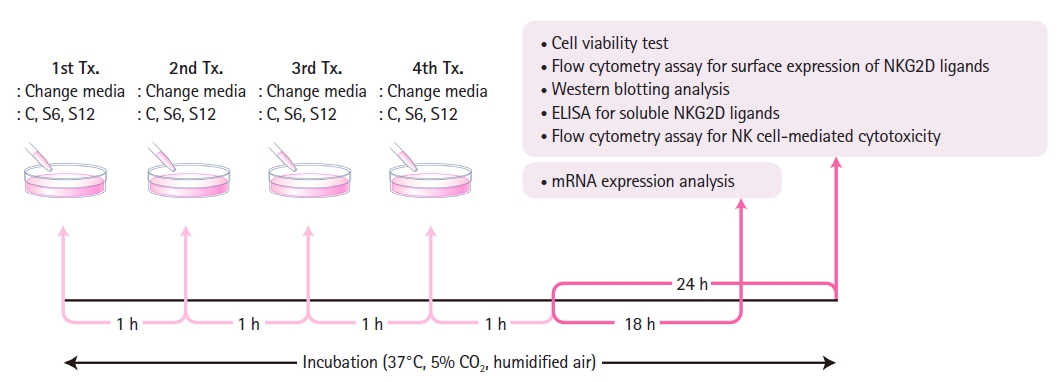
Fig. 2.
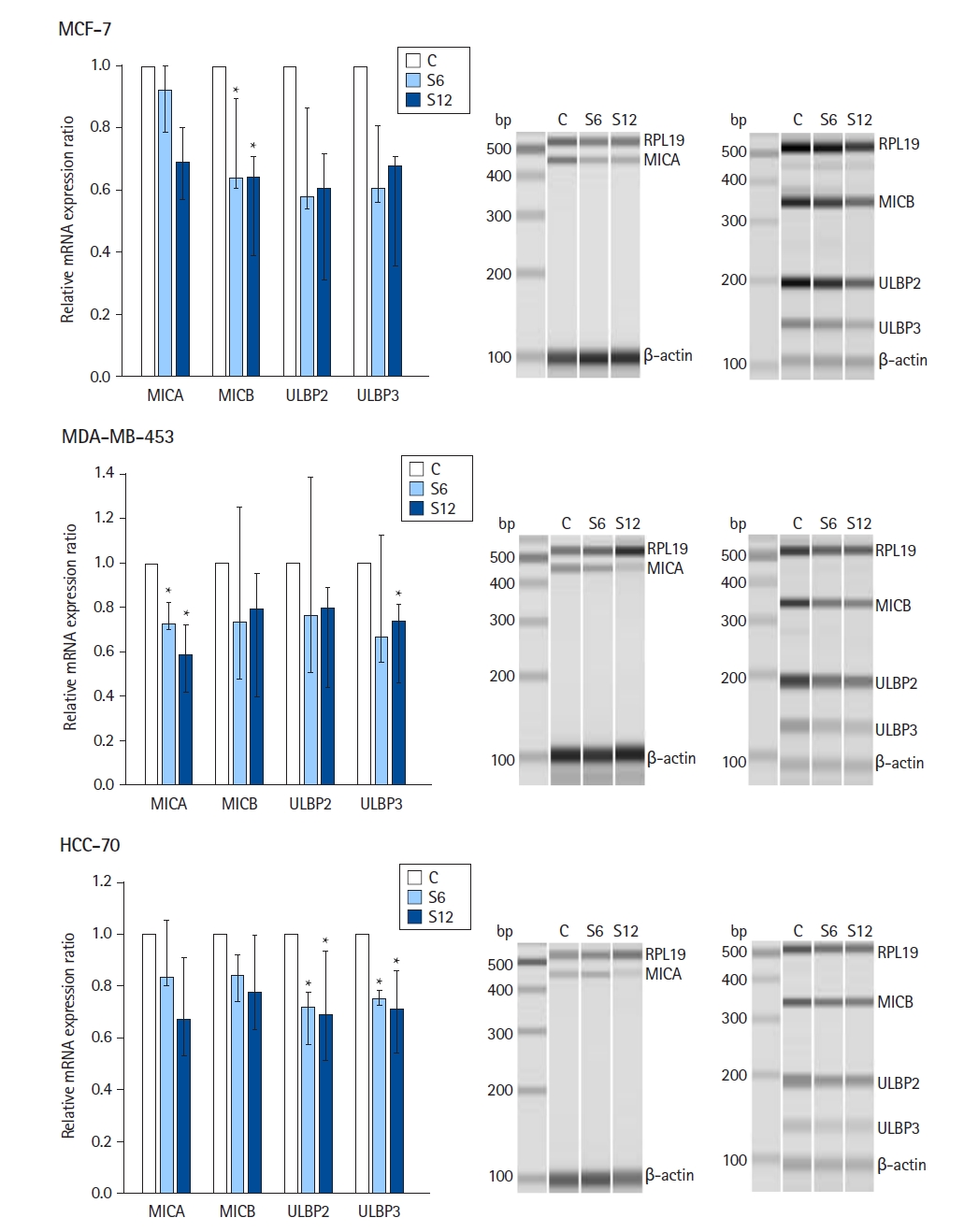
Fig. 3.
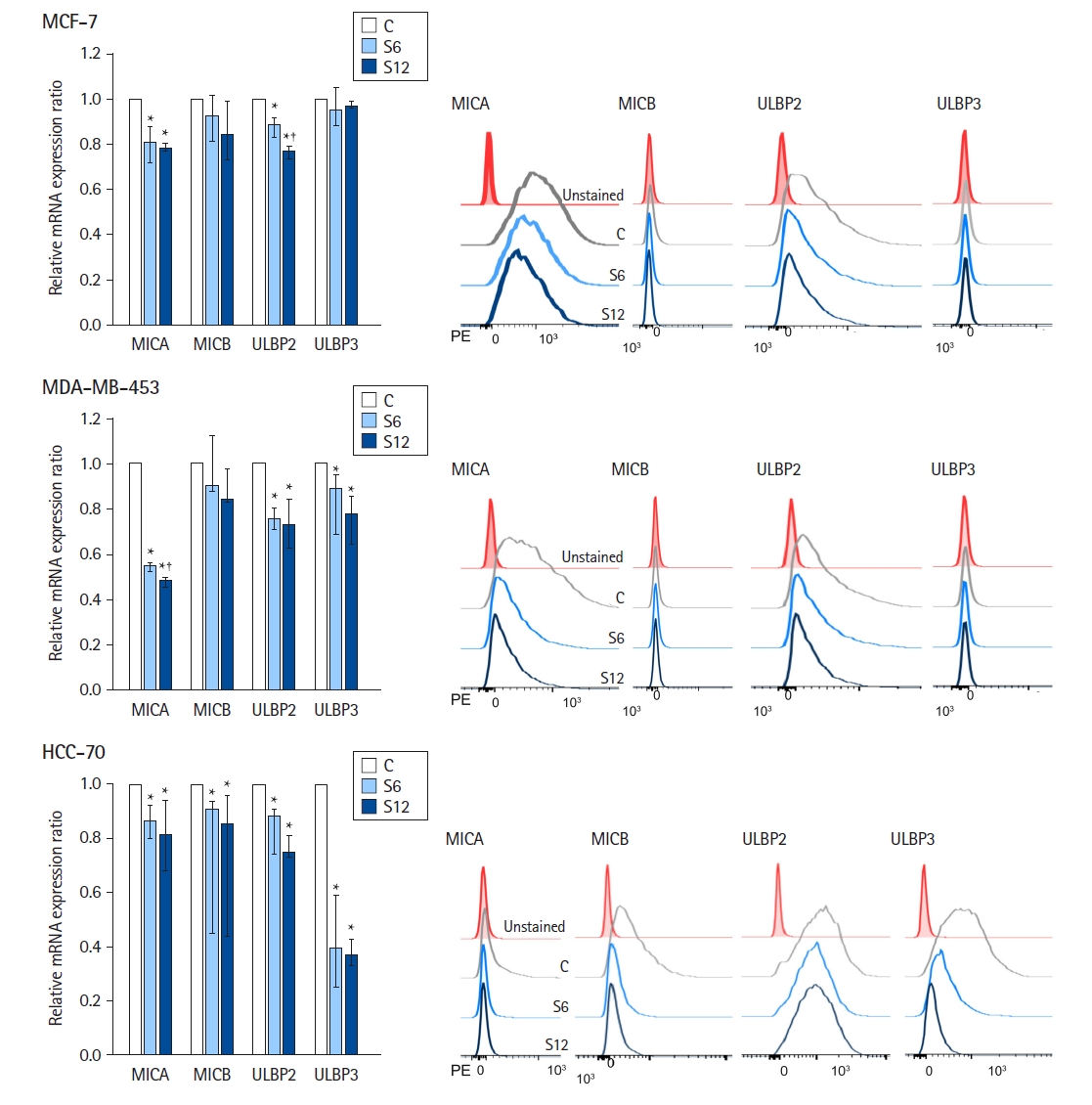
Fig. 4.
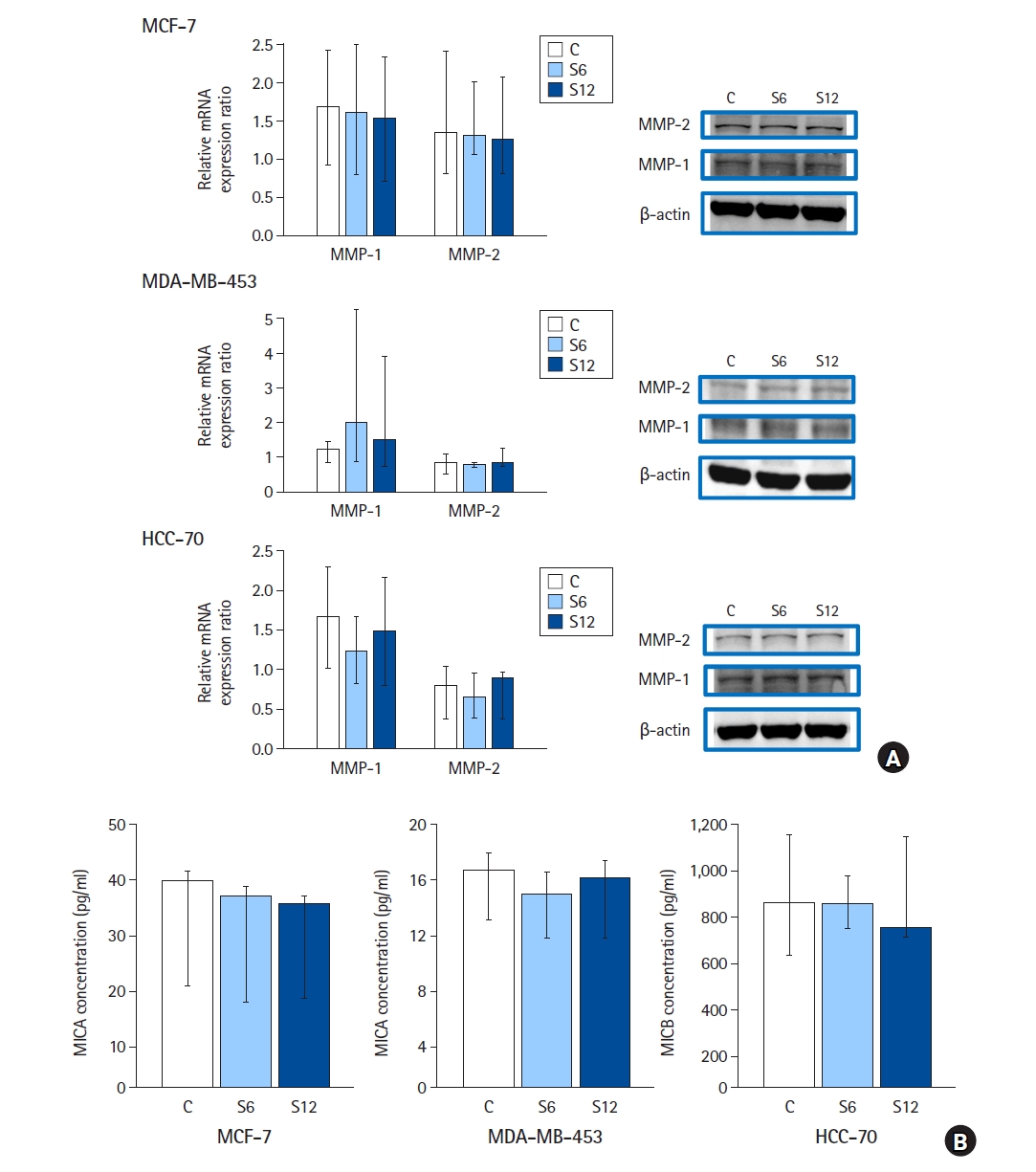
Fig. 5.










