|
 |
|
|
Abstract
Intracardiac thrombosis is an infrequent and fatal complication in patients with an inserted pacemaker. A patient with an inserted pacemaker scheduled for ureter stone removal experienced cardiac arrest and cardiopulmonary resuscitation under general anesthesia. Echocardiography showed multiple intracardiac thrombi. Preoperative diagnostic workup including echocardiography for the detection of pacemaker lead thrombus, and the need for anticoagulation should be considered in patients with an inserted pacemaker and high-risk factors for thrombosis.
The use of a cardiac pacemaker in patients with bradyarrhythmias is one of the most reliable and increasingly used treatments. It has been reported that nearly 3 million patients worldwide have a pacemaker [1]. Patients may require one or more surgical procedures after receiving the pacemaker, and surgical preparation should focus on safety. Although pacemaker insertion is generally considered safe, serious thrombotic and embolic complications occur in approximately 0.6-3.5% of cases [2]. We present a case of cardiac arrest associated with intracardiac thrombosis in a patient with a pacemaker during ureteroscopic lithotripsy for ureter stone under general anesthesia.
A 70-year-old woman with a five-year history of diabetes mellitus required ureter stone removal with general anesthesia. The height and weight of the patient were 153 cm and 59.6 kg, respectively.
Four months previous she received a dual pacing, dual sensing and dual response pacemaker for the treatment of second-degree heart block. At that time, transthoracic echocardiography (TTE) showed mild left ventricular hypertrophy and normal left ventricular ejection fraction (EF = 74%) with no regional wall motion abnormalities. The pacemaker was checked by a cardiologist one month previous. She had received no anticoagulant therapy.
A follow-up electrocardiogram (ECG) showed normal sinus rhythm and laboratory studies were unremarkable. On consultation with a cardiologist, the patient was evaluated to have low Class II with 0.9% complications on Revised Cardiac Risk Index for the operation.
The patient was not premedicated. The intraoperative monitoring of the patient included non-invasive blood pressure, ECG (leadII, aVL), pulse oximetry (SpO2), Bispectral index (BIS), and end-tidal CO2 concentration (EtCO2) measurements. Initial blood pressure was 160/80 mmHg. Heart rate was 82 beats/min, and SpO2 was 99%. Anesthesia was induced using mask administration of 100% O2. Fentanyl 50 ┬Ąg, 2% lidocaine 40 mg, propofol 100 mg and rocuronium 40 mg were administered intravenously, and the trachea was intubated using a 7.0 mm cuffed endotracheal tube. Anesthesia was maintained using 2 L/min O2, 2 L/min nitrous oxide, and 6 vol% desflurane.
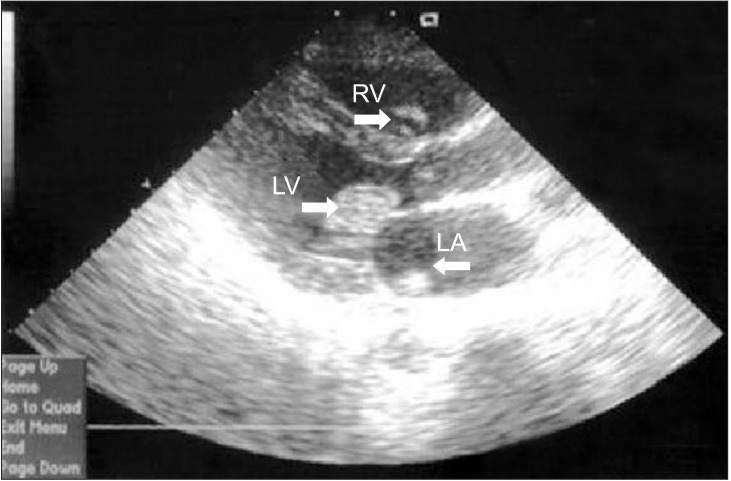
Ureteroscopic lithotripsy was performed in the lithotomy position. Blood pressure was maintained with systole 100-90 mmHg, diastole 60-50 mmHg, heart rate 60-55 beats/min and SpO2 99%. After approximately 40 minutes, SpO2 was suddenly not detected, BIS decreased from 45 to 19, blood pressure was not checked. EtCO2 dropped from 30 to 11, and both pupils dilatated without light reflex. ECG monitored the pacemaker's rhythm. External cardiac massage was immediately initiated and 1 mg doses of epinephrine and 0.5 mg doses of atropine were administered. TTE was immediately examined and showed full of multiple thrombi in all the cardiac chambers. Urokinase and two additional doses of epinephrine (1 mg) were administered, after which most thrombi dissolved or embolized via aorta (Fig. 1). Defibrillation was performed using 200 J, when ECG revealed ventricular fibrillation (VF). The patient was not resuscitated and died, despite continued cardiopulmonary resuscitation for about 60 minutes.
Rozmus et al. [3] reported that pacemaker lead can cause thrombus formation. Clinically silent lead thrombosis occurs in up to 35-45% of all patients with implanted pacemaker. However, pacemaker lead-associated intracardiac thrombus formation is a rare, but fatal condition [2]. A recent autopsy series for patients with implantable cardiac devices identified pulmonary emboli in 21% of patients [4]. Coleman et al. [5] reported intracardiac thrombosis associated with transvenous pacemaker leads in 24 patients. However, a case of intracardiac thromboembolism was not reported yet in patients with an inserted pacemaker having surgical procedure other than surgery for insertion of pacemaker or defibrillator.
Minimal invasive surgical procedures, such as shockwave lithotripsy and ureteroscopy, exhibit a low risk of thromboembolism, and do not typically require pharmacological antithrombotic therapy [6]. Preoperative anticoagulation can be considered for patients with an implanted pacemaker with high-risk diseases such as atrial fibrillation (AF) or mechanical heart valves [7]. Our patient was not administered with anticoagulation therapy after pacemaker insertion, but a compression elastic stocking to prevent thromboembolism was used without pharmacologic thromboprophylaxis during ureteroscopic lithotripsy.
Primary risk factors for pulmonary embolism (PE) include: deficiencies of antithrombin III, proteins C or S. Secondary risk factors comprise the history of deep vein thrombosis (DVT) or PE, immobilization, pregnancy, lower limb fracture, heart failure, obesity, cancer, a surgical operation lasting longer than 30 minutes, patients older than 40 years of age, and intraoperative leg elevation [8]. This patient had several risk factors including; 1) surgical operation lasting longer than 30 min; 2) older than 40 years of age and; 3) intraoperative leg elevation, for DVT and PE. Furthermore, general anesthesia tends to trigger decreased ventricular contractibility and more hypercoagulable states. Otherwise, it is reported that regional anesthetic technique reduces the risk of DVT. In our case, she received general anesthesia because we could not exclude possibility of conversion to open surgery and patient refused regional anesthesia.
Also, pacemaker lead insertion state can be a risk factor associated with DVT because the flow change of the affected vein and endothelial trauma create a prothrombotic state and vessel occlusion [3]. Therefore, in our patient, DVT could have been a possible cause of cardiac thromboembolism. Several study groups have attempted to find the risk factors for thromboembolism among the patients implanted with cardiac pacing devices. Korkeila et al. [9] reported that the absence of anticoagulant or antiplatelet therapy, multiple leads, and personal history of DVT to be predictive of thrombosis among patients implanted with cardiac pacing devices.
The diagnostic workup for lead thrombus detection is necessary for scheduled surgery for patients with pacemaker; venography, a CT scan or transesophageal echocardiography (TEE) can be useful. A diagnosis of thrombus should be considered in pacemaker implantation patients who are hemodynamically compromised. It is more likely to follow a rapid course, leading to death within several hours. So, prompt diagnosis and management may decrease morbidity and mortality [10].
Although TEE has greater sensitivity to detect thrombus, TTE could also be used bedside diagnostic tool to detect intracardiac thrombi [11]. In this case, we performed TTE. Multiple thrombi were observed in all heart chambers. We assumed that these thrombi may be caused by pacemaker leads, and a coagulant trend associated with cardiac arrest accelerated thrombus development. The formation of intracardiac thrombus is known to complicate several low-flow cardiac states, including severe acute myocardial infarction, AF, and severe cardiomyopathy, which can lead to life-threatening circulatory arrest [12].
Procoagulant activity after cardiac arrest and cardiopulmonary resuscitation tend to intensify with time, even in patients without preexisting coagulation abnormalities [13]. If thrombus is confirmed, pharmacological anticoagulation, thrombolysis, or surgical removal should be considered for treatment [4]. Most thrombi dissolved upon administration of urokinase. However, cardiac arrest continued.
There have been periprocedural management guidelines about preoperative interruption of oral anticoagulation (OAC) and heparin bridging for patients with an implanted pacemaker with AF or mechanical heart valves. But, there are no clear guidelines of using prophylactic anticoagulation for patients with pacemaker who are scheduled for surgical procedure and have not had any previous anticoagulation therapy. Nevertheless, anticoagulation therapy need to be considered because the pacemaker leads likely represented a significant thrombotic risk factor [14].
For AF patients with a low risk of thromboembolic events, simple interruption of OAC, without the use of heparin bridging is typically recommended. However, for AF patients with a higher risk of thromboembolism including mechanical mitral valves, guidelines recommend bridging with low molecular weight or unfractionated heparin in order to minimize the amount of time the patient is without anticoagulation. The study about safety and effectiveness of heparin bridging and continued oral anticoagulation is currently in progress [15].
Balancing the risk of thromboembolism against bleeding risk makes it so difficult to decide anticoagulation therapy for the patients scheduled for surgery. Because pacemaker implantation can be an important risk factor for thromboembolism during surgery along with other risk factors the patient may present, preoperative diagnostic evaluation for DVT and the decision to start anticoagulation are very necessary.
In conclusion, preoperative diagnostic workup and anticoagulation must be considered in high-risk patients with implanted pacemaker, even when minimal invasive procedures present low risk for thromboembolism. Intraoperative careful monitoring and early detection modality of embolism, such as TEE, is necessary, as is aggressive and immediate treatment for intracardiac thromboembolism.
References
1. Rozner MA. The patient with a cardiac pacemaker or implanted defibrillator and management during anaesthesia. Curr Opin Anaesthesiol 2007; 20: 261-268. PMID: 17479032.


2. Barakat K, Robinson NM, Spurrell RA. Transvenous pacing lead-induced thrombosis: a series of cases with a review of the literature. Cardiology 2000; 93: 142-148. PMID: 10965084.


3. Rozmus G, Daubert JP, Huang DT, Rosero S, Hall B, Francis C. Venous thrombosis and stenosis after implantation of pacemakers and defibrillators. J Interv Card Electrophysiol 2005; 13: 9-19. PMID: 15976973.


4. D'Aloia A, Bonadei I, Vizzardi E, Curnis A. Right giant atrial thrombosis and pulmonary embolism complicating pacemaker leads. BMJ Case Rep 2013; 2013.

5. Coleman DB, DeBarr DM, Morales DL, Spotnitz HM. Pacemaker lead thrombosis treated with atrial thrombectomy and biventricular pacemaker and defibrillator insertion. Ann Thorac Surg 2004; 78: e83-e84. PMID: 15511419.


6. Bourdoumis A, Stasinou T, Kachrilas S, Papatsoris AG, Buchholz N, Masood J. Thromboprophylaxis and bleeding diathesis in minimally invasive stone surgery. Nat Rev Urol 2014; 11: 51-58. PMID: 24346006.



7. Jamula E, Douketis JD, Schulman S. Perioperative anticoagulation in patients having implantation of a cardiac pacemaker or defibrillator: a systematic review and practical management guide. J Thromb Haemost 2008; 6: 1615-1621. PMID: 18638011.


8. Lee JY, Lee SY, Shin I, Park C, Lee BS, Kim MS. Fatal pulmonary embolism and coincidental cerebral infarction after spinal anesthesia-A case report. Korean J Anesthesiol 2011; 61: 515-518. PMID: 22220231.



9. Korkeila P, Mustonen P, Koistinen J, Nyman K, Ylitalo A, Karjalainen P, et al. Clinical and laboratory risk factors of thrombotic complications after pacemaker implantation: a prospective study. Europace 2010; 12: 817-824. PMID: 20348141.



10. Desciak MC, Martin DE. Perioperative pulmonary embolism: diagnosis and anesthetic management. J Clin Anesth 2011; 23: 153-165. PMID: 21377083.


11. Korkeila PJ, Saraste MK, Nyman KM, Koistinen J, Lund J, Juhani Airaksinen KE. Transesophageal echocardiography in the diagnosis of thrombosis associated with permanent transvenous pacemaker electrodes. Pacing Clin Electrophysiol 2006; 29: 1245-1250. PMID: 17100678.


12. Budhram GR, Mader TJ, Lutfy L, Murman D, Almulhim A. Left ventricular thrombus development during ventricular fibrillation and resolution during resuscitation in a swine model of sudden cardiac arrest. Resuscitation 2014; 85: 689-693. PMID: 24518559.


13. Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Bottiger BW, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008; 118: 2452-2483. PMID: 18948368.


14. Spar DS, Anderson JB, Palumbo JS, Kukreja KU, Czosek RJ. Symptomatic upper-extremity deep venous thrombosis after pacemaker placement in a pediatric patient: how to treat? Pediatr Cardiol 2013; 34: 1275-1279. PMID: 22618585.


15. Healey JS, Brambatti M. Periprocedural management of oral anticoagulation in patients with atrial fibrillation: approach in the era of new oral anticoagulants. Can J Cardiol 2013; 29(7 Suppl): S54-S59. PMID: 23790599.


- TOOLS