|
 |
|
|
Abstract
Because of insufficient number of donor hearts for cardiac transplantation, the use of implantable left ventricular assist device (LVAD) has been increasing as an alternative. During this procedure, the fundamental role of anesthesiologists would be to maintain stable hemodynamics. This report describes the anesthetic case of a 75-year-old man who underwent implantable LVAD placement as a destination therapy of his heart failure in Korea. The procedure and anesthesia were uneventful with transesophageal echocariographic guide. He moved to the ward on postoperative day 10 without fatal complication.
Since the first human trial in the mid-1980s, the use of left ventricular assist devices (LVADs) has gradually increased for bridging therapy to cardiac transplantation. In addition, the introduction of small pump with continuous flow, which is widely used at present, has significantly improved the prognosis after insertion and also has reduced the risk of thromboembolism [1]. Therefore, the role of LVAD has expanded into the destination therapy in patients with end-stage heart failure [2]. However, anesthesia for LVAD insertion is still challenging to the clinicians because hypotensive crisis is frequently developed and because it is sometimes difficult to maintain sufficient LVAD flow, especially in the case of massive bleeding [3]. Moreover, the careful and precise examination of transesophageal echocardiography (TEE) is essential for surgical decision. In this case report, we would like to introduce our anesthetic experience with HeartMate II LVAD (Thoratec, Pleasanton, CA, USA) insertion.
A 75-year-old man (height, 165 cm; weight, 49.5 kg) was admitted for the medically-untreatable congestive heart failure. At 63 years of age, he underwent the aortic valve replacement with Medtronic Hall valve (Medtronic, Inc, Minneapolis, MN, USA) due to severe aortic regurgitation of rheumatic origin. A year after the operation, congestive heart failure was induced by tachycardia. Acute renal failure was wax and wane, according to his condition. He was taking warfarin and platelet aggregation inhibitor. His prothrombin time was 1.52 INR and platelet count was 7,5000/┬Ąl. Implantable LVAD insertion was decided as a better therapeutic option than cardiac transplantation because of his old age.
Dobutamine (3 ┬Ąg/kg/min) had been continuously administered to stabilize vital sign from the ward. On arrival at the operating room, his blood pressure (BP) was 87/55 mmHg and the heart rate was 55 beats/min with atrial fibrillation. An invasive arterial catheter was placed in a radial artery with local anesthetic infiltration. Anesthesia was induced with 20 mg of etomidate, 50 mg of rocuronium, 50 ┬Ąg of sufentanil and maintained with 1-2% isoflurane-medical air-O2 (fraction of inspired oxygen, 50%). BP immediate after the tracheal intubation (Table 1) was 70/40 mmHg, but overcame within 3 min after using 20 mg of ephedrine, 20 ┬Ąg of epinephrine and increasing in dose of continuous dobutamine infusion up to 10 ┬Ąg/kg/min. Tidal volume was set at 8-10 ml/kg of ideal body weight and respiratory rate was adjusted 6-14/min to maintain arterial carbon dioxide tension at 35-45 mmHg. Mechanical ventilation was continued at 5 cycles/min with tidal volume of 5ml/kg of ideal body weight during cardiopulmonary bypass (CPB) for preventing atelectasis and ischemia reperfusion injury to the lung [4]. A pulmonary arterial catheter was inserted through the right internal jugular vein to monitor pulmonary artery pressure and obtain hemodynamic profiles, such as cardiac output and mixed venous O2 saturation, and placement of a TEE probe was performed.
His left ventricular (LV) ejection fraction was 20% by Simpson's method and the prosthetic valve was well-functioning on TEE. Severely dilated LV internal diameter was 69 mm at the end of contraction and mitral valve tethering with mitral regurgitation was observed. Before initiating CPB, atrial septal defect or patent foramen ovale was evaluated by TEE using agitated saline injection. Agitated saline test was done with pulmonary artery squeezing and confirmed absence of the right to the left shunt.
Redosternotomy was extended into the upper abdomen. Blood pump pocket was secured below the diaphragm within the abdominal muscles. Outflow graft was anastomosed to the side of the proximal ascending aorta with partial clamping. Activated clotting time (ACT) was 688 sec after 150 mg of heparin administration and CPB flow rate was maintained with 4.2 L/min. Maze operation for atrial fibrillation was also implemented and the mechanical aortic valve was closed by over-sewing both sides of the valve with dacron patches. Using circular blade, LV apex was perforated parallel to the septum, and apical ring was completely inserted into the LV apex after apical muscle resection. Inflow conduit was put into the apical ring and prepared purse string suture enabled the ring tightened.
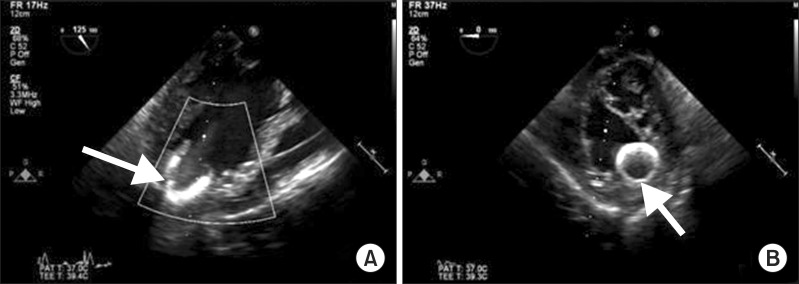
After implantation, TEE showed the correct placement of inflow cannula near the LV apex (Fig. 1) and no evidence of mitral regurgitation or right ventricular (RV) dysfunction. Twenty parts per million (PPM) of inhaled nitric oxide, 0.38 ┬Ąg/kg/min of milrinone, and 2 ┬Ąg/kg/min of isoproterenol were started to use before CPB weaning for preventing RV dysfunction after LVAD placement. In addition, 5 ┬Ąg/kg/min of dobutamine, 0.2 mcg/kg/min of norepinephrine and 6 IU/h of vasopressin were used to manage hypotension and to prevent vasoplegia. After weaning from CPB, protamine 120 mg was slowly injected and ACT was checked 117 sec. Surgeons, anesthesiologists and perfusionists closely communicated volume status to maintain sufficient LVAD flow because of massive bleeding. Volume status was evaluated overall by a visual inspection of the heart, TEE and mixed venous O2 saturation. Estimated bleeding was 5000 ml and 7 units (U) of red blood cell (RBC), 5 U of fresh frozen plasma, 23 U of cryoprecipitate, and 2 U of plateletpheresis concentrate were transfused. A total of 4 gm of tranxaminic acid was injected.
After surgery, he was transferred to the intensive care unit, and ongoing bleeding from the chest tube was 2 L during the first 3hours of admission. Coagulation studies identified no severe coagulopathy showing INR 1.42, ACT 135 sec, fibrinogen 236 mg/dl and platelet count 74,000/┬Ąl. Reoperation was immediately performed and LV apical site bleeding was successfully controlled. Extubation was done at 15 hours after the reoperation and he was moved to the general ward on postoperative day 10, without significant complications.
Implantable LVADs have been adopted for patients with end stage heart failure requiring hospital-based support or a heart transplant. Severely impaired cardiac function has been known to increase the risk for perioperative morbidity and mortality, and even minor changes in hemodynamics will lead to catastrophic response that is hard to get over. After implantation, normovolemia should be guaranteed for adequate device function [3] even in massive bleeding. Therefore, anesthesia for LVADs implantation needs considerable effort to maintain stable vital signs. In our case, etomidate was used as an anesthetic agent to prevent hypotension during induction because of its advantageous cardiovascular profile [5]. Etomidate was reported as anesthetic induction agent in 81% of patients undergoing implantable LVADs [6]. Moreover, when anesthesia induces, inotropics and vasopressors should be prepared to support hemodynamic stabilization. Low dose vasopressin infusion was proven to prevent postbypass vasoplegia and decrease vasopressors requirement when started to administer before CPB [3,7]. TEE or pulmonary artery catheter should be used for monitoring adequate volume status in the procedure [8].
As our case, patients presenting LVADs insertion are usually in high risk for coagulopathy. Systemic anticoagulation is in general use to avoid formation of intracardiac thrombus when low cardicac output state exists. In addition, deficiency in coagulation factors results from congestive hepatic failure as a complication of cardiac failure [6]. Transfusion of RBCs has demonstrated to worsen the surgical outcome and so antifibrinolytics or recombinant factor VII might be considered to treat coagulopathy and to decrease aggressive transfusion [9,10].
TEE plays an essential role for LVAD implantation. Before initiating CPB, evaluation by TEE should concentrate on detecting abnormalities of the insertion site, right to the left shunt, and valvular function [11]. Access to proper location for LVADs is ruling out ascending aortic atherosclerosis and ventricular thrombus or scar. Hypoxemia would appear during CPB weaning if the right to left shunt remained unrepaired. Decreased outflow will be influenced by severe aortic insufficiency. In our case, the mechanical aortic valve was occluded owing to increased risk of thromboembolism [6] in condition of non-pulsatile blood pumping. In addition, after weaning from CPB, TEE is very helpful for checking the correct alignment of cannulas, unobstructed flow pattern, and volume status.
RV failure has been proven to be related with poor outcome after LVAD placement [12]. It has not been clarified with the exact reason why RV dysfunction happens after LVADs placement, although the loss of interventricular contribution on right ventricle might be regarded as a possible reason [13]. It could result in LV preload decrease, and consequently, disturb successful operation of LVADs. Dobutamine and milrinone might be effective to avoid RV failure [14] and inhaled nitric oxide could be beneficial to decrease RV afterload [15].
In conclusion, the surgery for the placement of implantable LVAD is expected to increase in Korea. During this procedure, anesthesiologists should be focused on preventing right ventricular failure and maintaining normovolemia. Knowing the detailed surgical procedure might be essential for successful management. In addition, the meticulous examination of TEE would be very useful during the entire procedure.
References
1. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361: 2241-2251. PMID: 19920051.


2. Garbade J, Bittner HB, Barten MJ, Mohr FW. Current trends in implantable left ventricular assist devices. Cardiol Res Pract 2011; 290561PMID: 21822483.




3. Feussner M, Mukherjee C, Garbade J, Ender J. Anaesthesia for patients undergoing ventricular assist-device implantation. Best Pract Res Clin Anaesthesiol 2012; 26: 167-177. PMID: 22910088.


4. Ng CS, Arifi AA, Wan S, Ho AM, Wan IY, Wong EM, et al. Ventilation during cardiopulmonary bypass: impact on cytokine response and cardiopulmonary function. Ann Thorac Surg 2008; 85: 154-162. PMID: 18154801.


5. Ray DC, McKeown DW. Etomidate in critically ill patients. Pro: yes, we can use it. Eur J Anaesthesiol 2012; 29: 506-510. PMID: 22907611.


6. Broussard D, Donaldson E, Falterman J, Bates M. Anesthesia for left ventricular assist device insertion: a case series and review. Ochsner J 2011; 11: 70-77. PMID: 21603338.


7. Hasija S, Makhija N, Choudhury M, Hote M, Chauhan S, Kiran U. Prophylactic vasopressin in patients receiving the angiotensin-converting enzyme inhibitor ramipril undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2010; 24: 230-238. PMID: 19875309.


8. Kartha V, Gomez W, Wu B, Tremper K. Laparoscopic cholecystectomy in a patient with an implantable left ventricular assist device. Br J Anaesth 2008; 100: 652-655. PMID: 18344554.



9. Kuitunen A, Hiippala S, Vahtera E, Rasi V, Salmenper├ż M. The effects of aprotinin and tranexamic acid on thrombin generation and fibrinolytic response after cardiac surgery. Acta Anaesthesiol Scand 2005; 49: 1272-1279. PMID: 16146463.


10. Warren O, Mandal K, Hadjianastassiou V, Knowlton L, Panesar S, John K, et al. Recombinant activated factor VII in cardiac surgery: a systematic review. Ann Thorac Surg 2007; 83: 707-714. PMID: 17258029.


11. Chumnanvej S, Wood MJ, MacGillivray TE, Melo MF. Perioperative echocardiographic examination for ventricular assist device implantation. Anesth Analg 2007; 105: 583-601. PMID: 17717209.


12. Drakos SG, Janicki L, Horne BD, Kfoury AG, Reid BB, Clayson S, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol 2010; 105: 1030-1035. PMID: 20346326.


13. Damiano RJ Jr, La Follette P Jr, Cox JL, Lowe JE, Santamore WP. Significant left ventricular contribution to right ventricular systolic function. Am J Physiol 1991; 261: H1514-H1524. PMID: 1951739.


14. Aranda JM Jr, Schofield RS, Pauly DF, Cleeton TS, Walker TC, Monroe VS Jr, et al. Comparison of dobutamine versus milrinone therapy in hospitalized patients awaiting cardiac transplantation: a prospective, randomized trial. Am Heart J 2003; 145: 324-329. PMID: 12595851.


15. Kukucka M, Potapov E, Stepanenko A, Weller K, Mladenow A, Kuppe H, et al. Acute impact of left ventricular unloading by left ventricular assist device on the right ventricle geometry and function: effect of nitric oxide inhalation. J Thorac Cardiovasc Surg 2011; 141: 1009-1014. PMID: 20884019.


- TOOLS