 |
 |
|
|
Abstract
Background
Clonidine has been shown to be a potent neuroprotectant by acting at α2 receptors on glutamatergic neurons to inhibit the release of glutamate. The aim of this study is to investigate the effects of clonidine on the activity of EAAT3 that can regulate extracellular glutamate.
Methods
EAAT3 was expressed in the Xenopus oocytes. Using a two-electrode voltage clamp, membrane currents were recorded after application of 30 µM L-glutamate both in the presence and absence of various concentrations of clonidine. To determine the effects of clonidine on the Km and Vmax of EAAT3 and the reversibility of clonidine effects, membrane currents were recorded after the application of various concentrations of L-glutamate both in the presence and absence of 1.50 × 10-7 M clonidine.
Results
Clonidine reduced the EAAT3 responses to L-glutamate in a concentration-dependent manner. This inhibition was statistically significant at higher concentrations than at the clinically relevant range. Clonidine at 1.50 × 10-7 M reduced the Vmax, but did not affect the Km of EAAT3 for L-glutamate.
Conclusions
These results suggest that the direct inhibition of EAAT3 activity is not related to the sedation effect of clonidine and that the clonidine-induced reduction of EAAT3 activity provides additional data for the possible involvement of glutamatergic hyperactivity in the proconvulsant effect of clonidine.
L-Glutamate is the major excitatory neurotransmitter in the central nervous system (CNS) and plays a dominant role in central excitatory neurotransmission involved in neuronal survival, synaptogenesis, neuronal plasticity, learning and memory processes. Glutamate is found naturally in millimolar levels in the brain; however, it can cause excitotoxic neuronal damage at high extracellular concentrations [1]. Extracellular glutamate concentrations are maintained within physiological levels by glutamate transporters (also known as excitatory amino acid transporters, EAATs) because no extracelluar enzymes exist for the breakdown of glutamate [2]. Five major subtypes of EAATs have been identified. EAAT1 is present in glial cells throughout the CNS and at high levels in the Bergmann glia of the cerebellum. EAAT2 is almost exclusively glial, and is widespread and highly abundant throughout the forebrain, cerebellum and spinal cord. The EAAT3 and EAAT4 are present predominantly in neurons; EAAT3 is selectively enriched in the neurons of the hippocampus, basal ganglia, cerebellum, and olfactory bulb, whereas EAAT4 is predominantly localized to cerebellar Purkinje cells, with low levels of expression also present in the forebrain. EAAT5 is present in the rod photoreceptor and bipolar cells of the retina [3,4].
Astroglial glutamate transporters (EAAT1 and EAAT2) are the predominant pathway for the synaptic inactivation of glutamate in the forebrain. Although there is little evidence for presynaptic or postsynaptic inactivation of glutamate by neuronal transporters, at least in the hippocampus and cerebral cortex, glutamate uptake could be primarily neuronal because many of the synapses are not surrounded by astrocytic processes in these brain areas [5]. Several studies have reported that EAAT3 activity increases with volatile anesthetics [6], propofol [7], lidocaine [8] and benzodiazepines [9], which could explain the anesthetic actions and the neuroprotective and antiepileptic effects of these drugs.
Clonidine, a selective α2-adrenergic receptor agonist, exhibits anesthetic, sedative, anxiolytic, sympatholytic and analgesic properties in clinical practice [10]. It has also been shown that clonidine is a potent neuroprotector in various cerebral hypoxia-ischemia models in which the accumulation of high concentrations of glutamate in the extracellular space is a key pathologic event and that α2-adrenergic receptors are important in mediating clonidine-induced neuroprotection [11,12]. It has been reported that activation of α2-adrenergic receptors located on the nerve terminals of the glutamatergic neurons inhibit the release of glutamate [13]. Considering these results, the sedative, anesthetic, and analgesic actions as well as the neuroprotective effect of clonidine may be implicated in the glutamatergic system, including the effect on the activity of the glutamate transporter. However, it is not known whether clonidine can affect the EAAT3 activity. The aim of this study is to investigate the effects of clonidine on the activity of EAAT3 proteins that can regulate extracellular glutamate using Xenopus oocytes.
Mature female Xenopus laevis frogs were purchased from Kato S Science (Chiba, Japan) and fed regular frog brittle twice weekly. All reagents, unless specified below, were obtained from Sigma (St. Louis, MO, USA). For removal of the oocytes, the frogs were anesthetized in 500 ml 0.2% 3-aminobenzoic acid ethyl ester in water until unresponsive to painful stimuli (toe pinching) and underwent surgery on ice. The oocytes were surgically retrieved and placed immediately in a calcium-free OR-2 solution. The OR-2 solution comprised (in mM): 82.5 NaCl, 2 KCl, 1 MgCl2, 5 HEPES with pH adjusted to 7.5. The oocytes were defolliculated with gentle shaking for approximately 2 hours in a calcium-free OR-2 solution including 0.1% collagenase type Ia. The oocytes were then incubated in a modified Barth's solution at 16℃ for one day before the injection of EAAT3 mRNA. The modified Barth's solution comprised (in mM): 88 NaCl, 1 KCl, 2.4 NaHCO3, 0.41 CaCl2, 0.82 MgSO4, 0.3 Ca (NO3)2, 0.1 gentamicin and 15 HEPES, with pH adjusted to 7.5.
The rat EAAT3 complementary DNA (cDNA) construct was provided by Dr. Mattias A. Hediger (Brigham and Women's Hospital, Harvard Institutes of Medicine, Boston, MA, USA). The cDNA was subcloned in a commercial vector (BluescriptSKm). The plasmid DNA was linearized with a restriction enzyme (Not I) and mRNA was synthesized in vitro with a commercially available kit (Ambion, Austin, TX, USA). The resulting mRNA was quantified spectrophotometrically and diluted in sterile water. This mRNA was used for the cytoplasmic injection of oocytes in a concentration of 40 ng/ 40 nl by using an automated microinjector (Nanoliter 2000; World Precision Instruments, Sarasota, FL, USA). The oocytes were then incubated at 16℃ in a modified Barth's solution for three days before voltage-clamping experiments.
Experiments were performed at room temperature (approximately 21-23℃). A single oocyte was placed in a recording chamber that was < 1 ml in volume and was perfused with 5 ml Tyrode's solution/min. The Tyrode's solution comprised (in mM): 150 NaCl, 5 KCl, 2 CaCl2, 1 MgSO4, 10 dextrose and 10 HEPES, with pH adjusted to 7.5. Clamping microelectrodes were pulled from capillary glass (Single-Barrel Standard Borosilicate Glass Tubing, World Precision Instruments) on a micropipette puller (Temperature controlled pipette puller PIP5; HEKA Instruments Inc., Bellmore, New York, USA). The tips were broken at a diameter of approximately 10 µm and filled with 3 M KCl, obtaining a resistance of 1-3 MΩ. The oocytes were voltage-clamped using a two-electrode voltage clamp amplifier (OC725-A; Warner Corporation, New Haven, CT, USA) that was connected to a data acquisition and analysis system running on a personal computer. The acquisition system consisted of a DAS-8A/D conversion board (Keithley-Metrabyte, Taunton, MA, USA). Analyses were performed with pCLAMP7 software (Axon Instruments, Foster City, CA, USA). All measurements were performed at a holding potential of -70 mV. The oocytes that did not show a stable holding current of less than 1 µA were excluded from analysis. L-Glutamate was diluted in Tyrode's solution and superfused over the oocyte for 25 s (5 ml/min). The L-Glutamate-induced inward currents were sampled at 125 Hz for 1 min: 5 s of baseline, 25 s of L-glutamate application and 30 s of washing with Tyrode's solution. The glutamate-induced peak currents were calculated to reflect the amount of glutamate transported. We used 30 µM L-glutamate, unless indicated otherwise, in this study because the value of the pharmacokinetic parameter Km of EAAT3 for L-glutamate was shown to be 27-30 µM in previous studies [6,14].
Clonidine hydrochloride was dissolved in dimethyl sulfoxide and then diluted by Tyrode's solution to the appropriate final concentrations (0.2, 0.5, 2, 4, 7, 10, 20, 40 or 50 ng/ml that corresponds to 7.50 × 10-10, 1.88 × 10-9, 7.50 × 10-9, 1.50 × 10-8, 2.63 × 10-8, 3.75 × 10-8, 7.50 × 10-8, 1.50 × 10-7 or 1.88 × 10-7 M), which include clinically relevant plasma concentrations. In the control experiments, the oocytes were perfused with Tyrode's solution for 4 min before the application of Tyrode's solution containing L-glutamate for the electrophysiological recording. In the clonidine-treated group, the oocytes were perfused with Tyrode's solution for the first minute for stabilization followed by Tyrode's solution containing clonidine for 3 min before the application of Tyrode's solution containing L-glutamate for the electrophysiological recording. To determine the effects of clonidine on the Km and Vmax of EAAT3 and the reversibility of clonidine effects, the responses to serial concentrations of L-glutamate (3, 10, 30, 100, 300 and 1,000 µM) were assayed, the oocytes were then treated with 1.50 × 10-7 M clonidine and the responses were again recorded. For the stabilization, the oocytes were perfused with Tyrode's solution for 4 min before the second and third measurements.
Responses are reported as mean ± SEM. Each experimental condition was performed with the oocytes from at least three different frogs. Since the expression level of the transporter proteins in the oocytes of different batches may vary, variability in responses among the batches of oocytes is common. Thus, the responses were normalized to the mean value of the sameday controls for each batch. Similarly, in the reversibility experiments, the responses were normalized to the responses of the same oocytes to 1 mM glutamate under control conditions (before the clonidine treatment). This concentration of glutamate was the highest concentration used to induce EAAT3 activity and this normalization allowed us to pool together data from different batches of oocytes for analysis. Statistical analysis was performed using the Student's t-test, one-way repeated measures analysis of variance (ANOVA) or one way ANOVA followed by the Tukey multiple comparison test (SPSS version 18.0, SPSS Inc., an IBM Company, Chicago, IL, USA) as appropriate. Differences were considered significant at P < 0.05. Dose-response curves were prepared and IC50 was calculated using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA).
The oocytes injected with EAAT3 mRNA showed inward currents after L-glutamate application (Fig. 1), whereas the uninjected oocytes were unresponsive to L-glutamate (data not shown). This current has been shown to be mediated via EAAT3 in previous studies [4,14].
In the vehicle control experiment, 0.04% (v/v) of dimethyl sulfoxide, the solvent for clonidine, (the highest concentration in Tyrode's solution containing clonidine) had no effect on the current response to glutamate (0.97 ± 0.11-fold) compared with the control (1.00 ± 0.13-fold) (n = 10, P > 0.05).
While clonidine itself did not induce any current in the oocytes injected with or without EAAT3 mRNA (data not shown), clonidine reduced the EAAT3 responses to L-glutamate (Fig. 1) in a concentration-dependent manner (7.50 × 10-10 - 1.88 × 10-7 M). The IC50 value (concentration required for 50% inhibition) of clonidine was 2.72 × 10-8 M. This inhibition was statistically significant at 2.63 × 10-8 - 1.88 × 10-7 M. Since the inhibition reached maximal at 1.50 × 10-7 M, we used this concentration for further experiments.
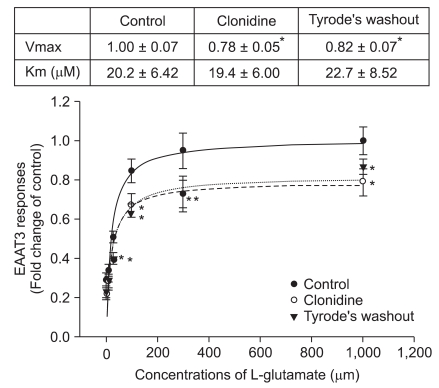
The Km of EAAT3 for L-glutamate was 20.2 ± 6.42 µM. This Km was not significantly affected by 1.50 × 10-7 M clonidine. However, clonidine significantly decreased the Vmax of EAAT3 for L-glutamate (from 1.00 ± 0.07 of control group to 0.78 ± 0.05 of clonidine group, P < 0.05). This clonidine effect decreased slightly, but not significantly in the oocytes perfused with Tyrode's solution for 4 min after clonidine treatment (Fig. 2).
We demonstrated that clonidine did not have a significant effect on the activity of EAAT3, a major neuronal glutamate transporter, at clinically relevant concentrations (1.88 × 10-9 - 1.50 × 10-8 M, corresponding to 0.5-4 ng/ml), but that clonidine concentration-dependently reduced the EAAT3 activity significantly at higher concentrations (2.63 × 10-8 - 1.88 × 10-7 M, corresponding to 7-50 ng/ml).
EAATs are sodium co-transporters. They co-transport two or three sodium ions with one negatively charged glutamate molecule into the cell. Thus, at least one net positive charge enters the cell per glutamate transported. Thus, the transport of glutamate is electrogenic, and the size of the glutamate-induced current reflects the amount of glutamate transported. Therefore, measuring the glutamate-induced currents has been used widely in the literature to quantify EAAT activity [15]. Considerable evidence suggests that volatile, intravenous and local anesthetics may affect the EAAT3 activity [6-9,16].
Clonidine is an imidazoline derivate and a mixed α1 and α2 adrenoreceptor agonist with a predominant α2 action [17]. More recently, clonidine has been assuming greater importance as an anesthetic adjuvant because of its sedative, anxiolytic and analgesic properties in adult and pediatric practice [10]. The sedative/anesthetic-sparing properties of α2-adrenergic agonists are ascribable to the actions in the locus ceruleus, which is associated with a variety of physiologic regulatory processes, including regulation of sleep and wakefulness [17].
Arenas-López et al. [18] demonstrated a consistent and adequate level of sedation in the majority of cases at clonidine plasma concentrations of 0.9-2.5 ng/ml. Sumiya et al. [19] reported that the plasma clonidine concentration of 0.3-0.8 ng/ml would be sufficient to produce a satisfactory sedation in pediatric surgery. Clonidine is also known to be effective in the treatment of attention deficit-hyperactivity disorder at a therapeutic range of 0.5-4.5 ng/ml [20]. The exact clonidine concentrations around EAAT3 in the brains of patients on therapeutic doses of clonidine are not known. Clonidine crosses the blood brain barrier and the cerebrospinal fluid (CSF) concentration is 50% of the plasma concentration [21]. Thus, the therapeutic range of clonidine concentrations in the CSF may be lower than the clinically relevant plasma concentrations. Therefore, our results suggest that EAAT3 may not be a target for the sedative, anxiolytic, and anesthetic effects of clonidine.
We originally expected that clonidine may increase the EAAT3 activity because previous studies showed that volatile anesthetics, benzodiazepines, propofol and lidocaine increased the EAAT3 activity [6-9]. However, our results showed that clonidine rather reduced the EAAT3 activity significantly at 2.63 × 10-8 - 1.88 × 10-7 M (7-50 ng/ml). These concentrations are higher than the clinically relevant concentrations.
There is an apparent inconsistency in the literature concerning the proconvulsant, anticonvulsant and no effects of clonidine [22-24]. One of the working mechanisms of clonidine is the down-regulation of norepinephrine release form the locus ceruleus. It is known that the neurotransmitter norepinephrine plays a relevant role in modulating seizures [25]. Although interactions between central norepinephrine disturbances and seizure susceptibility are not fully understood, there is some evidence that norepinephrine may act as an anticonvulsant via the augmentation of the inhibitory γ-aminobutyric acid (GABA) effect [26]. The inhibition of neuronal EAATs induces epilepsy in rats and decreases the inhibitory post-synaptic current mediated by GABA in the rat hippocampus via a reduced synthesis of GABA because glutamate taken up by neuronal EAATs is a substrate for GABA synthesis [27]. Several studies on experimental animals indicated that the effects of clonidine change according to dose. Some studies have found an anticonvulsant effect of low-dose clonidine, whereas high doses decreased seizure thresholds [28,29]. Although a direct or indirect effect via another system(s) cannot be excluded, the glutamate accumulation due to a decreased EAAT3 activity may be suggested as one of the possibilities of the proconvulsant effect of clonidine.
We also demonstrated that clonidine did not affect the Km, but reduced the Vmax of EAAT3 for L-glutamate, suggesting that clonidine does not affect the affinity of EAAT3 for L-glutamate but decreases the total EAAT3 available for glutamate transporting. This regulatory mechanism of EAAT3 is consistent with the previous studies with regard to other drugs, such as volatile anesthetics [6], propofol [7], lidocaine [8], ethanol [14] and amitriptyline [30]. The clonidine-induced decreased EAAT3 activity was slightly, but not fully, recovered after a short washout, indicating that the clonidine effect may be irreversible. We could not clarify the exact reason for this in this study. It is believed that further study is needed with a longer duration of washout to determine the reversibility of clonidine effects. Possible limitations of this study are that the experiment was in vitro and that the mRNA of the rat instead of the human mRNA was used.
In conclusion, clonidine showed an insignificant effect on the activity of EAAT3 at clinically relevant concentrations, but decreased the EAAT3 activity significantly at higher concentrations. These results suggest that the inhibition of EAAT3 activity is not related to the sedation effect of clonidine. However, these results may provide additional data for the possible involvement of glutamatergic hyperactivity in the proconvulsant effect of clonidine.
Acknowledgements
The study protocol (Ethical Committee no. MRI 10-03) was approved by the Ewha Medical Research Institute at the School of Medicine, Ewha Womans University, Seoul, Korea (Chairperson Prof. Seikwan Oh) on 29 March 2010.
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this article.
References
1. Bakuridze K, Savli E, Gongadze N, Baş DB, Gepdiremen A. Protection in glutamate-induced neurotoxicity by imidazoline receptor agonist moxonidine. Int J Neurosci 2009; 119: 1705-1717. PMID: 19922382.


2. O'Shea RD. Roles and regulation of glutamate transporters in the central nervous system. Clin Exp Pharmacol Physiol 2002; 29: 1018-1023. PMID: 12366395.


4. Maragakis NJ, Rothstein JD. Glutamate transporters in neurologic disease. Arch Neurol 2001; 58: 365-370. PMID: 11255439.


5. Bergles DE, Diamond JS, Jahr CE. Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol 1999; 9: 293-298. PMID: 10395570.


6. Do SH, Kamatchi GL, Washington JM, Zuo Z. Effects of volatile anesthetics on glutamate transporter, excitatory amino acid transporter type 3: the role of protein kinase C. Anesthesiology 2002; 96: 1492-1497. PMID: 12170065.


7. Do SH, Ham BM, Zuo Z. Effects of propofol on the activity of rat glutamate transporter type 3 expressed in Xenopus oocytes: the role of protein kinase C. Neurosci Lett 2003; 343: 113-116. PMID: 12759177.


8. Do SH, Fang HY, Ham BM, Zuo Z. The effects of lidocaine on the activity of glutamate transporter EAAT3: the role of protein kinase C and phosphatidylinositol 3-kinase. Anesth Analg 2002; 95: 1263-1268. PMID: 12401608.


9. Palmada M, Böhmer C, Centelles JJ, Kinne RK. Effect of benzodiazepine on the epithelial and neuronal high-affinity glutamate transporter EAAC1. J Neurochem 1999; 73: 2389-2396. PMID: 10582598.


10. Kamibayashi T, Maze M. Clinical uses of alpha2-adrenergic agonists. Anesthesiology 2000; 93: 1345-1349. PMID: 11046225.


11. Laudenbach V, Mantz J, Lagercrantz H, Desmonts JM, Evrard P, Gressens P. Effects of alpha(2)-adrenoceptor agonists on perinatal excitotoxic brain injury: comparison of clonidine and dexmedetomidine. Anesthesiology 2002; 96: 134-141. PMID: 11753013.


12. Hoffman WE, Cheng MA, Thomas C, Baughman VL, Albrecht RF. Clonidine decreases plasma catecholamines and improves outcome from incomplete ischemia in the rat. Anesth Analg 1991; 73: 460-464. PMID: 1897770.


13. Kamisaki Y, Hamahashi T, Okada CM, Itoh T. Clonidine inhibition of potassium-evoked release of glutamate and aspartate from rat cortical synaptosomes. Brain Res 1991; 568: 193-198. PMID: 1814567.


14. Kim JH, Lim YJ, Ro YJ, Min SW, Kim CS, Do SH, et al. Effects of ethanol on the rat glutamate excitatory amino acid transporter type 3 expressed in Xenopus oocytes: role of protein kinase C and phosphatidylinositol 3-kinase. Alcohol Clin Exp Res 2003; 27: 1548-1553. PMID: 14574224.


15. Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci 1994; 14: 5559-5569. PMID: 7521911.



16. Yun JY, Kim JH, Kim HK, Lim YJ, Do SH, Zuo Z. Effects of intravenous anesthetics on the activity of glutamate transporter EAAT3 expressed in Xenopus oocytes: evidence for protein kinase C involvement. Eur J Pharmacol 2006; 531: 133-139. PMID: 16413532.


17. Maze M, Tranquilli W. Alpha-2 adrenoceptor agonists: defining the role in clinical anesthesia. Anesthesiology 1991; 74: 581-605. PMID: 1672060.


18. Arenas-López S, Riphagen S, Tibby SM, Durward A, Tomlin S, Davies G, et al. Use of oral clonidine for sedation in ventilated paediatric intensive care patients. Intensive Care Med 2004; 30: 1625-1629. PMID: 15197439.


19. Sumiya K, Homma M, Watanabe M, Baba Y, Inomata S, Kihara S, et al. Sedation and plasma concentration of clonidine hydrochloride for pre-anesthetic medication in pediatric surgery. Biol Pharm Bull 2003; 26: 421-423. PMID: 12673018.


20. Farooqi M, Seifert S, Kunkel S, Johnson M, Benson B. Toxicity from a clonidine suspension. J Med Toxicol 2009; 5: 130-133. PMID: 19655285.



21. Lowenthal DT. Pharmacokinetics of clonidine. J Cardiovasc Pharmacol 1980; 2(Suppl 1): S29-S37. PMID: 6154834.


22. Feron FJ, Hendriksen JG, Nicolai J, Vles JS. Newonset seizures: a possible association with clonidine? Pediatr Neurol 2008; 38: 147-149. PMID: 18206800.


23. Papanicolaou J, Summers RJ, Vajda FJ, Louis WJ. Anticonvulsant effects of clonidine mediated through central alpha2-adrenoceptors. Eur J Pharmacol 1982; 77: 163-166. PMID: 6277661.


24. Yokoyama M, Hirakawa M, Goto H. Clonidine does not affect lidocaine seizure threshold in rats. Can J Anaesth 1993; 40: 1205-1209. PMID: 8281598.


25. Giorgi FS, Ferrucci M, Lazzeri G, Pizzanelli C, Lenzi P, Alessandrl MG, et al. A damage to locus coeruleus neurons converts sporadic seizures into self-sustaining limbic status epilepticus. Eur J Neurosci 2003; 17: 2593-2601. PMID: 12823466.


26. Cheun JE, Yeh HH. Modulation of GABAA receptor-activated current by norepinephrine in cerebellar Purkinje cell. Neuroscience 1992; 51: 951-960. PMID: 1283212.


27. Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R, et al. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J Neurosci 2002; 22: 6372-6379. PMID: 12151515.



28. Papanicolaou J, Summers RJ, Vajda FJ, Louis WJ. The relationship between alpha 2-adrenoreceptor seletivity and anticonvulsant effect in a series of clonidine-like drugs. Brain Res 1982; 241: 393-397. PMID: 6286046.


29. Jackson HC, Dickinson SL, Nutt DJ. Exploring the pharmacology of the pro-convulsant effects of alpha 2-adrenoreceptor antagonists in mice. Psychopharmacology (Berl) 1991; 105: 558-562. PMID: 1685254.


30. Baik HJ, Lee SA, Washington JM, Zuo ZY. Amitriptyline inhibits the activity of the rat glutamate transporter EAAT3 expressed in Xenopus oocytes. J Pharm Pharmacol 2009; 61: 577-581. PMID: 19405995.



Fig. 1
Dose-response of clonidine inhibition of EAAT3 responses to 30 µM L-glutamate. The IC50 value for this inhibition was 2.72 × 10-8 M. The upper graphs show typical current traces induced by the application of 30 µM L-glutamate. Data show the means ± SEM (n = 25-50 in each data point). *P < 0.05 compared to control.

Fig. 2
Effects of clonidine on EAAT3 activity. The EAAT3 responses to various L-glutamate concentrations were measured before (control group) and immediately after clonidine treatment (clonidine group) and after a 4-min Tyrode's perfusion (Tyrode's washout). The Vmax and Km values of EAAT3 response to L-glutamate are listed in the table above the figure. Data show the means ± SEM (n = 9-17 in each data point). *P < 0.05 compared to the corresponding values in the control group.
- TOOLS








