Introduction
Cardiac complications have emerged as major adverse events after non-cardiac surgery [
1]. With advances in perioperative medicine, the incidence of major postoperative complication is decreasing [
2]. Such advances in perioperative medicine are beneficial in daily clinical practice but a need for relevant studies on new outcome measures has arisen. Unlike major adverse events during perioperative periods, which are well associated with long-term prognosis, the association of some of the adverse events with long-term prognosis still remains unclear. According to some studies, some of the events that were even considered spontaneously reversible could also affect long-term prognosis [
3,
4]. Previously, we hypothesized that a composite of cardiac events could reflect prognosis adequately and be used as an objective and reliable outcome measure in non-cardiac surgery. By defining perioperative adverse cardiac events (PACE) as a composite of myocardial infarction (MI), coronary revascularization, congestive heart failure, arrhythmic attack, acute pulmonary embolism, cardiac arrest, and stroke during the 30-day postoperative period, we demonstrated an association with one-year mortality after non-cardiac surgery [
5]. We used a large-scale cohort dataset of over 200,000 consecutive adult patients undergoing non-cardiac surgery, but it was still limited to a single center, which lacks generalization.
Recently, the concept of a distributed research network has been developed to overcome the privacy issue in generating multicenter patient-level data. This method uses standardized, deidentified, decentralized electronic medical records (EMR), and conducts clinical research only with combined summary statistics [
6,
7]. In this study, we investigated data from multiple institutions within a distributed research network and aimed to demonstrate the association between PACE and long-term mortality with further nationwide multicenter evidence.
Materials and Methods
We conducted a multicenter retrospective cohort study using a distributed research network without patient-level data sharing. This study was approved by the Institutional Review Board (IRB) of Ajou University Medical Center (AJIRB-MED-MDB-21-662), and the need for individual written informed consent was waived. The other six hospitals are affiliated with the Research Border Free Zone of Korea that accepts approval of the research organizing center in the study using deidentified Common Data Model (CDM) data. This study complied with the principles of the Declaration of Helsinki, 2013 and was compiled using the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Data sources
This study included 8,280,723 patients from Korea’s electronic medical records database. Patient-level EMR data were standardized and deidentified into the standard vocabulary of the Observational Medical Outcomes Partnership (OMOP) CDM [
8] and were stored in each hospital. The hospitals included Ajou University Medical Center (AUMC; 1994.01-2022.02; 2,873,443 patients), Kyung Hee University Hospital (KHMC; 2008.01-2022.02; 1,168,640 patients), Gandong Kyung Hee University Hospital (KHNMC; 2006.06-2021.10; 805,332 patients), Kangdong Sacred Heart Hospital (KDH; 2005.01-2021.10; 1,101,850 patients), Pusan National University Hospital (PNUH; 2011.02-2019.08; 1,753,001 patients), Gyeongsang National University Hospital (GNUH; 2009.10-2022.04; 626,663 patients), and Wonkwang University Hospital (WKUH; 1998.03-2018.12; 1,001,794 patients).
Study design
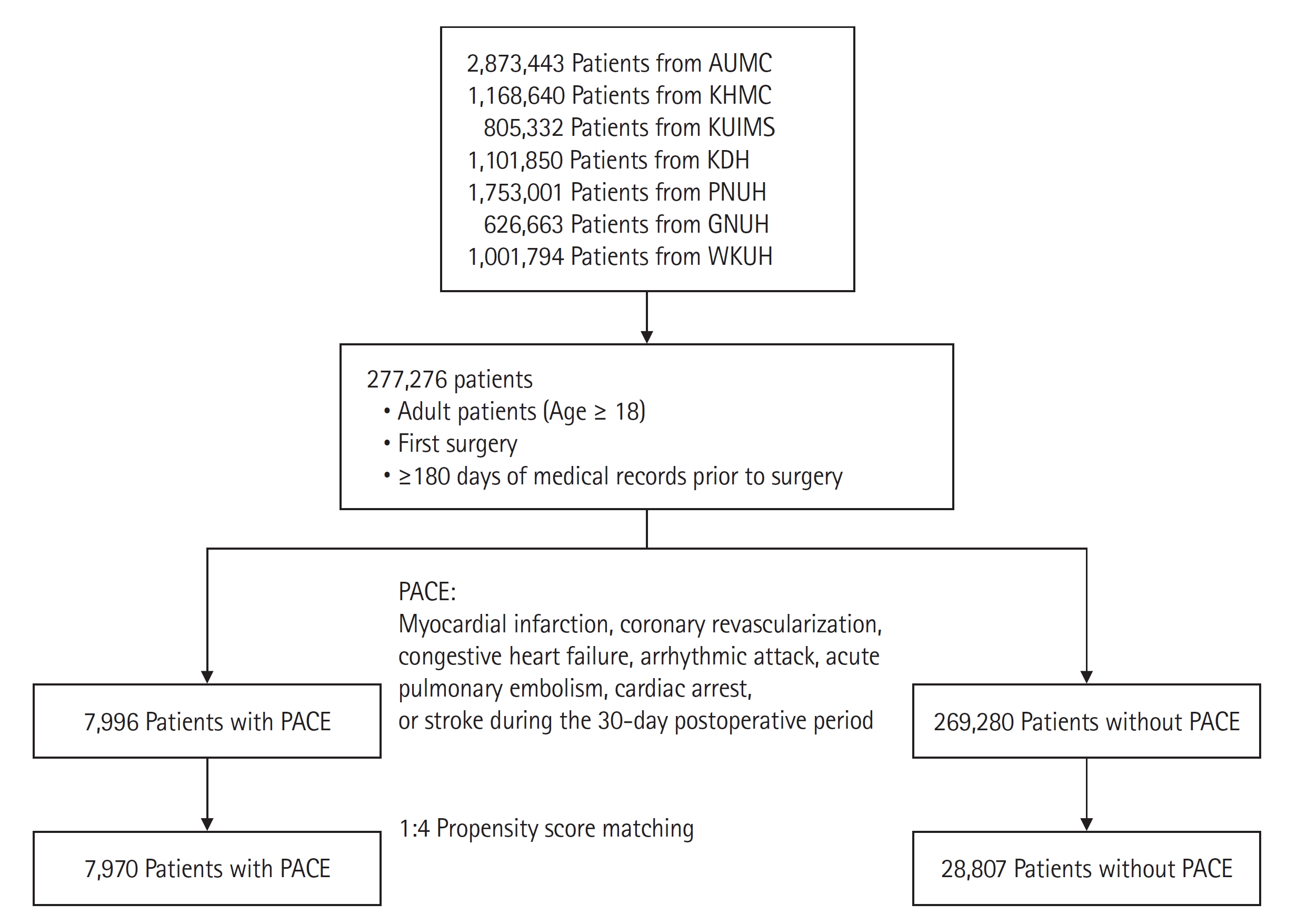
We extracted the records of 277,276 adult patients over 18 years of age who underwent surgery for the first time at the hospital and had medical records for more than 180 days before surgery. Because we planned to stratify patients according to the development of PACE during 30 days after surgery, those with mortality during the first 30 days after surgery were excluded from the study. Adult patients who underwent surgery before August 2018 at each hospital were recruited for a sufficient follow-up period.
Fig. 1 shows a flow chart of the study design.
We divided the patients into two groups in each hospital, according to PACE, defined as a composite of heart failure, arrhythmic attack, acute pulmonary embolism, cardiac arrest, MI, coronary revascularization, and stroke within 30 days of surgery. An arrhythmic attack was defined as rapid atrial fibrillation, ventricular tachycardia, and bradycardia that required medical intervention such as electrical shock, temporary cardiac pacing, or two or more consecutive administrations of antiarrhythmic agents. Cardiac arrest was defined as a diagnosis of cardiac arrest or a record of cardiopulmonary resuscitation. Pulmonary embolism was defined as the presence of a diagnostic code and an elevated d-dimer level. Coronary revascularization was confirmed by surgical records of coronary angiography using stent placement or coronary artery bypass graft surgery. MI was defined as the presence of a diagnostic code and a blood troponin level. Data on heart failure and stroke were obtained from the diagnostic codes, but only the first recorded diagnosis of each respective condition was included. The primary endpoint was one-year mortality, and the secondary endpoint was three-year mortality. Mortality data were extracted from the death certificates issued by each hospital.
We divided the patients into two groups in each hospital according to the occurrence of PACE and generated a 1:4 matched population with propensity score matching (PSM). We conducted a stratified Cox regression analysis in a matched population to calculate the hazard ratio (HR) of one-year and three-year mortality [
9].
After conducting survival analysis in a distributed research network, we aggregated the results of seven databases by meta-analysis. Then we conducted a subgroup analysis on emergency operation status and surgical risk, categorized according to the European Society of Cardiology/European Society of Anesthesiology guidelines on non-cardiac surgery. We also performed a subgroup analysis based on comorbidities such as diabetes, hypertension, and chronic kidney disease, and cancer. Furthermore, we performed subgroup analysis with different demographics, sex, and older age (> 65 years). When performing subgroup analysis, variables that divide the subgroup were excluded from the PSM variable.
Further, we categorized the PACE events into six subgroups, including heart failure, MI or revascularization, pulmonary embolism, cardiac arrest, arrhythmic attack, and stroke. For each event subgroup, we conducted survival analysis using Cox regression after performing 1:4 propensity matching separately, as done in the main analysis for PACE. We calculated the HRs for each hospital and conducted a meta-analysis to provide the point estimates, 95% CIs, and P values for the risk ratios for each event group.
In our analysis, age covariates were grouped by five years. The disease covariate was binarized as whether a patient was diagnosed with a specific International Classification of Diseases code in the last 365 days. Comorbidity was quantified using Romano’s adaptation of the Charlson Comorbidity Index [
10]. The risk of surgical procedure was classified according to European Society of Cardiology/European Society of Anesthesiology guidelines on non-cardiac surgery [
11].
Statistical analysis
For baseline characteristics, we calculated mean ± standard deviation or median with interquartile range (IQR) and the t-test or the Mann-Whitney
U test for continuous variables. For categorical variables, we calculated percentages and used the chi-square or Fisher’s exact test to compare differences between the groups. In PSM, we matched four patients without PACE to one patient with PACE and used 0.1 standardized logits as the caliper width. The PSM covariates were: sex, age, diabetes, chronic kidney disease, stroke, coronary artery disease, heart failure, arrhythmia, peripheral artery disease, aortic disease, valvular heart disease, current alcohol use, and Romano’s Adaptation of the Charlson comorbidity index [
10]. In subgroup analysis, variables that divide the subgroup are excluded from the PSM variable. After PSM, we regarded an absolute standard mean deviation (ASD) of 0.1 as balanced and calculated as HRs with 95% CIs using stratified Cox survival analysis.
In a meta-analysis, we conducted statistical tests of heterogeneity by using χ2 and I2 statistics. When I2 < 50%, we mainly reported a fixed-effects model. Otherwise, a random-effects model was used. All analyses were performed using R (version 4.1.0; R Foundation for Statistical Computing®).
To test for differences between subgroups, we adopted single variable meta-regression. Meta-regression is a method that considers confounding covariates while conducting meta-analysis. We conducted meta-regression with subgroup characteristics as a single variable. Then, we named the statistical significance of the subgroup variable as the ‘P value for difference’ and used it as a T-test for subgroup difference.
Results
In a total of 277,276 patients undergoing non-cardiac surgery, PACE was observed in 7,996 (2.88%) patients. After 1:4 PSM, 7,970 patients with PACE and 28,807 patients without PACE were matched. The baseline characteristics of the matched AUMC cohort are shown in
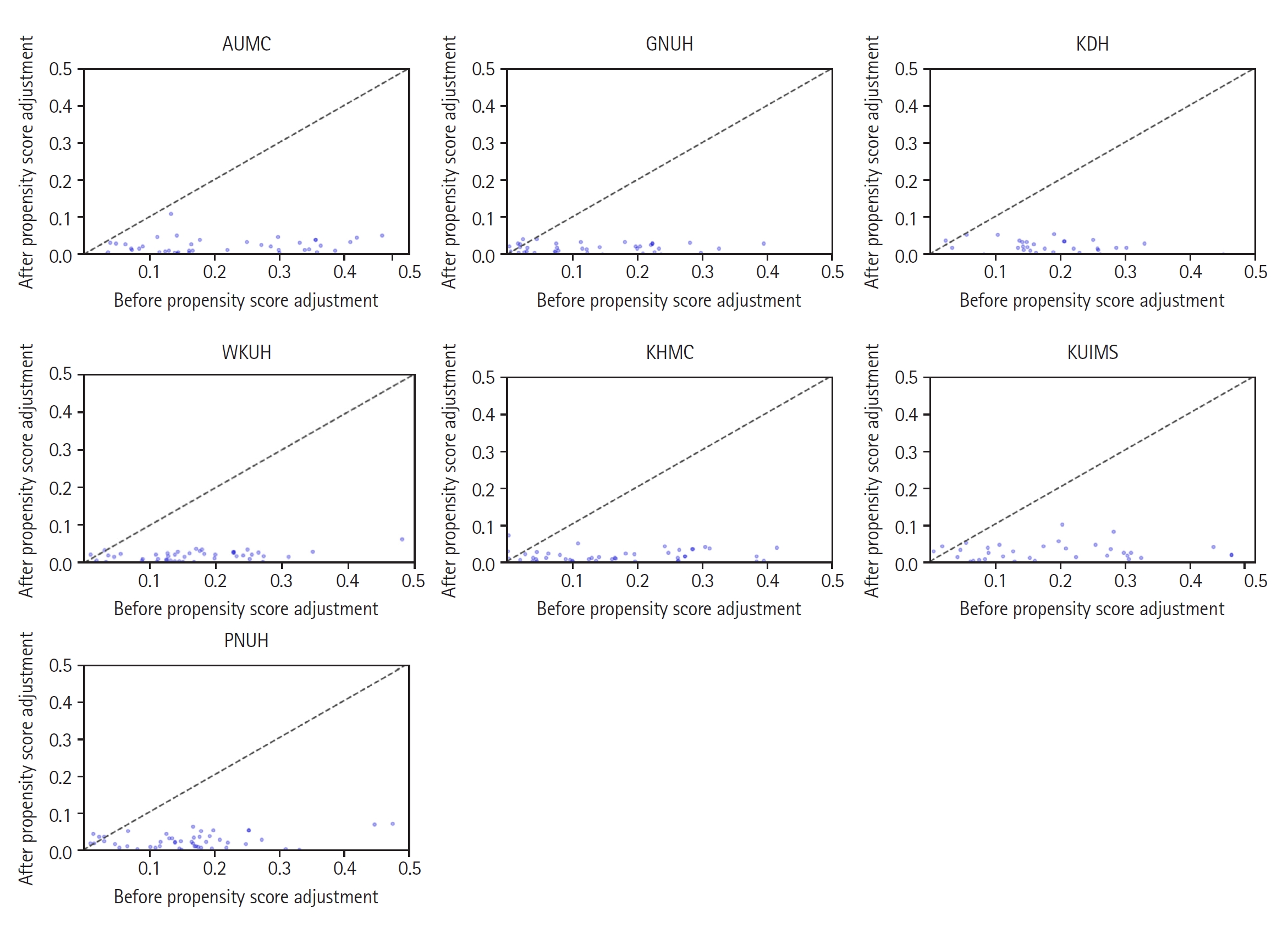
Table 1. The table shows that the PACE group was predominantly male and had a higher incidence of comorbidity. The baseline covariates became well-balanced after PSM (ASD < 0.1). The improvements in the balance between covariates in other cohorts are also shown as changes in ASD in
Fig. 2. Propensity score distribution according to the presence or absence of PACE for each hospital is described in
Supplementary Fig. 1.
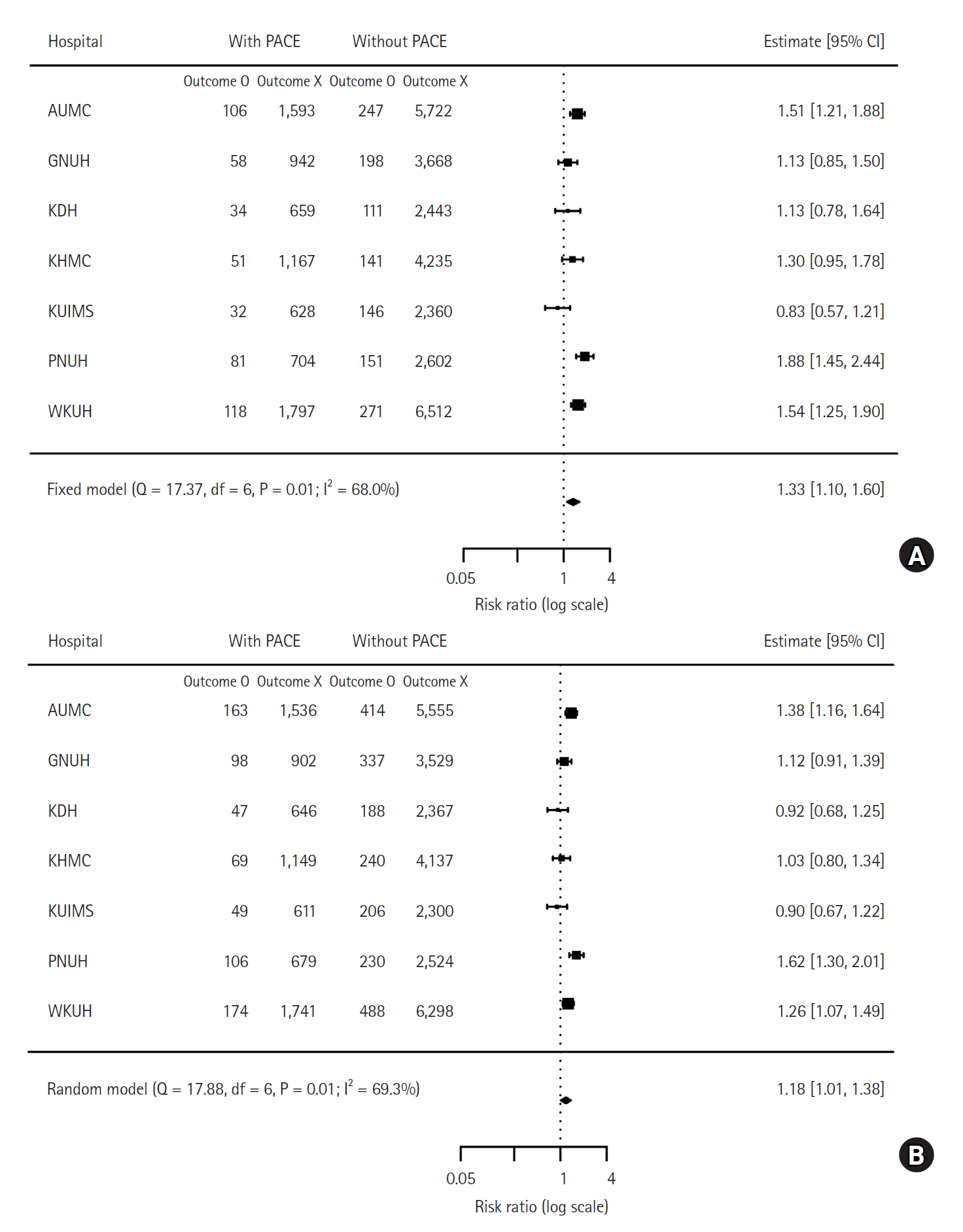
The number of deaths within one year and three years was 480 (6%) and 706 (8.6%) in patients with PACE, and 1,265 (4.4%) and 2,103 (7.3%) in those without PACE. The meta-analysis showed that PACE was associated with a higher risk of one-year mortality (HR: 1.33, 95% CI [1.10, 1.60], P = 0.005) and a higher risk of three-year mortality (HR: 1.18, 95% CI [1.01, 1.38], P = 0.038). The one-year and three-year mortality risks for each cohort are presented in a forest plot in
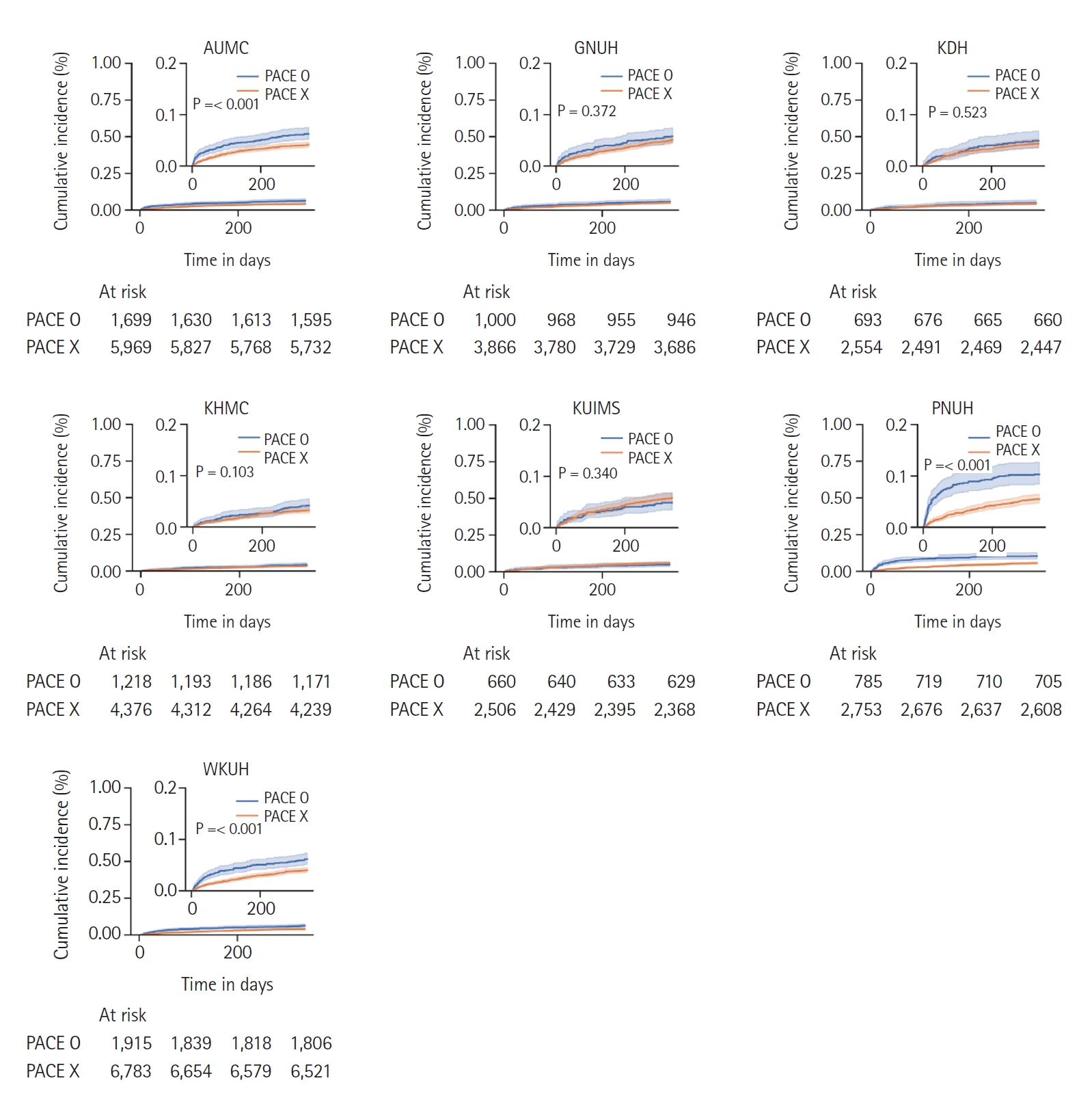
Fig. 3. Survival plots with one-year mortality in each cohort are shown in
Fig. 4.
The association between PACE and one-year mortality was analyzed separately according to surgical risk. The association was significant regardless of surgical risk, but the increase of risk for one-year mortality associated with PACE became higher as the risk of surgical risk became higher (HR: 1.20, 95% CI [1.04, 1.39], P = 0.020 for low-risk surgery; HR: 1.69, 95% CI [1.45, 1.96], P < 0.001 for intermediate-risk; and HR: 2.38, 95% CI [1.47, 3.86], P = 0.034 for high-risk). The forest plots for the risk of one-year mortality of each cohort are presented according to surgical risk in
Fig. 5. In all groups except for the patients without cancer and young age group, PACE was found to be statistically associated with a higher risk of mortality (HRs ranging from 1.20 to 2.38, 95% CIs ranging from 1.04 to 3.86). In the subgroup analysis, the P value for difference was 0.026 for age, 0.399 for sex, 0.063 for cancer, 0.601 for chronic kidney disease, 0.269 for diabetes, 0.110 for hypertension, 0.011 for emergency operation, and 0.011 for surgical risk. Based on these results, we have shown that there is a statistically significant risk in the older age group, emergency operation group, and high surgical risk group. Specifically, elderly patients (> 65 years) (HR: 1.89, 95% CI [1.30, 2.74]), and emergency surgery patients (HR: 1.74, 95% CI [1.42, 2.15]) had a higher risk of mortality compared to their counterparts. The detailed results of subgroup analysis are descripted in
Table 2. The detailed forest plot for each PACE event can be found in
Supplementary Fig. 2.
The event-specific analysis showed that except for stroke (HR: 1.22, 95% CI [0.90, 1.64], P = 0.194), all other PACE events were significantly associated with a higher risk of mortality. Specifically, HR for heart failure was 1.34 (95% CI [1.10, 1.65], P = 0.005), for pulmonary embolism HR was 2.33 (95% CI [1.40, 3.88], P = 0.001), for cardiac arrest HR was 14.42 (95% CI [6.46, 32.17], P < 0.001), for arrhythmic attack HR was 2.07 (95% CI [1.55, 2.76], P < 0.001), and for MI or revascularization HR was 1.51 (95% CI [1.17, 1.95], P = 0.002). The number of patients for each event for each hospital is provided in
Supplementary Table 1. We have summarized the results in
Supplementary Table 2. The detailed forest plot for each PACE event can be found in
Supplementary Fig. 3.
Discussion
This study demonstrates the association between PACE, a composite of MI, coronary revascularization, congestive heart failure, arrhythmic attack, acute pulmonary embolism, cardiac arrest, and stroke during a 30-day postoperative period, and mortality after non-cardiac surgery. We used a multicenter cohort and demonstrated the association between PACE and mortality after non-cardiac surgery, which was consistent with our previous report [
5].
Our results may be helpful in future clinical trials because we propose an optimal composite endpoint that is powerfully relevant to prognosis but not too rare. In clinical trials, there is an increased propensity to collate patient outcomes into one composite endpoint, including nonfatal complications, owing to a dramatic improvement in fatal outcomes [
12]. An endpoint with extremely low incidence requires a large number of study participants that makes clinical trials difficult to conduct, and is also problematic from an ethical perspective [
13]. To enhance the generalizability of the results, we analyzed nationwide multicenter data and further conducted subgroup analyses. In our subgroup analysis, PACE was found to be statistically associated with a higher risk of mortality in all groups except for the patients without cancer and young age group (HRs ranging from 1.20 to 2.38 with corresponding CIs ranging from 1.04 to 3.86). Moreover, the increase in the risk for mortality by PACE tended to be greater in high-risk patients such as the elderly and those who underwent higher-risk procedures. In addition, our data showed that an increase in the risk for mortality by PACE, shown as HR, tended to be greater in high-risk patients such as the elderly, those who underwent higher-risk procedures, or those with comorbidities. Considering that clinical trials generally target patients with certain risk factors, this finding indicates that PACE may be an optimal endpoint. Also, compared to major adverse cardiac events, which already have been used in previous studies, PACE contains arrhythmias that not uncommonly occurs and are associated with poor long-term outcomes after surgery. Therefore, PACE could provide a more accurate reflection of the overall risk of adverse cardiac events following non-cardiac surgery.
When interpreting an association shown in an observational study, the clinical relevance of the result should be carefully discussed. Most of the components in PACE were individually investigated in previous studies. PACE includes the components of major adverse cardiac events, a well-known composite endpoint for the clinical trial [
14], and the association with mortality for a major cardiac event is well established. On the other hand, the clinical relevance of some minor components of PACE, such as arrhythmic events, still needs to be clarified. Among arrhythmic events, postoperative atrial fibrillation is prevalent after non-cardiac surgery and is associated with increased morbidity and length of hospital stay [
15]. Most postoperative atrial fibrillation spontaneously reverses to a normal rhythm, but it still affects long-term mortality and stroke risk [
16,
17]. On the other hand, there is still limited data on the individual effects of different types of arrhythmias, and this may need further investigation.
The methodologic strength of this study is that we analyzed the deidentified cohort. Privacy has recently arisen as a major ethical issue in clinical studies, especially when using data from EMR [
18]. A privacy protection issue has made it more difficult for investigators to collect and analyze patient-level data for multicenter evidence. The concept of a distributed research network is a recent concept that was developed to overcome privacy issues in a multicenter observational study [
8]. In this method, standardized, deidentified, and decentralized EMRs were obtained and analyzed at each center. Instead of centralizing the data for analysis, the results are provided only as combined summary statistics.
The results of this study should be interpreted as descriptive due to the following limitations. As we analyzed a retrospective administrative dataset, unmeasured variables may not have been balanced even after rigorous statistical adjustments with PSM. Although we used multicenter datasets, they were all in Korea, and most of the study patients were Asian. Therefore, our analysis may not be generalizable in other regions and may show an ethnic difference. This study was conducted as a multi-center, decentralized retrospective study based on OMOP CDM. Due to the nature of the study, some specified information was not feasible that can lead to inconsistencies in the diagnostic criteria of PACE. To address this issue, we combined diagnostic codes with laboratory values, medication prescriptions, and procedural records to increase the reliability. However, inconsistencies in the diagnostic criteria of primary exposures may still exist. In further studies, that include information like imaging reports, transthoracic echocardiography should be included. Due to limited sample size and data availability issues, we could not include variables such as the type of surgery, emergency status, presence of cancer (although we included in the charlson comorbidity index), cancer stage, and functional status in the PSM. To alleviate this limitation, we conducted subgroup analyses based on the type of surgery, emergency status, and presence of cancer. However, due to the current limitations of the CDM, we were unable to include information on cancer stage and functional status. To address these limitations and conduct thorough multi-institutional databases, we need to connect more detailed postoperative information to OMOP CDM-based standardized databases. Lastly, perioperative care was not controlled between centers. Despite the presence of the perioperative guidelines, many clinical decisions may have been made at the clinician’s discretion, and over the long study period, some clinical guidelines may have changed. Despite these limitations, we demonstrated an association between PACE 30 days after non-cardiac surgery and one-year mortality in a nationwide multicenter clinical dataset. Our findings show that PACE may be a suitable composite endpoint for future clinical trials.
In non-cardiac surgery patients, an association of PACE with one-year mortality was observed in a nationwide multicenter study. A further prospective study regarding PACE as a composite endpoint that is relevant to prognosis is required.