Comparison of analgesic effects between programmed intermittent epidural boluses and continuous epidural infusion after cesarean section: a randomized controlled study
Article information
Abstract
Background
This study aimed to compare the analgesic effects of programmed intermittent epidural boluses (PIEB) and continuous epidural infusion (CEI) for postoperative analgesia after elective cesarean section (CS).
Methods
Seventy-four women who underwent elective CS were randomized to receive either PIEB or CEI. The PIEB group received 4 ml-intermittent boluses of 0.11% ropivacaine every hour at a rate of 120 ml/h. The CEI group received a constant rate of 4 ml/h of 0.11% ropivacaine. The primary outcome was the pain score at rest at 36 h after CS. Secondary outcomes included the pain scores during mobilization, time-weighted pain scores, the incidence of motor blockade, and complications-related epidural analgesia during 36 h after CS.
Results
The pain score at rest at 36 h after CS was significantly lower in the PIEB group compared with that in the CEI group (3.0 vs. 0.0; median difference, 2; 95% CI: 1, 2; P < 0.001). The mean time-weighted pain scores at rest and during mobilizations were also significantly lower in the PIEB group than in the CEI group (pain at rest: mean differences, 37.5; 95% CI, [24.6, 50.4]; P < 0.001; pain during mobilization: mean difference, 56.6; 95% CI, [39.8, 73.5]; P < 0.001). The incidence of motor blockade was significantly reduced in the PIEB group compared with that in the CEI group (P < 0.001).
Conclusion
PIEB provides superior analgesia with less motor blockade than CEI in postpartum women after CS, without any apparent adverse events.
Introduction
With the increasing rates of cesarean section (CS) worldwide, there has been growing interest among clinicians on the effective management of postoperative pain following CS [1]. Adequate postoperative pain control is crucial for promoting mobility, ensuring emotional well-being, and preventing chronic pain. Insufficient pain control in the postpartum period negatively affects maternal birth experiences and hinders newborn care and breastfeeding [2,3]. Given the major impact of postoperative pain on newborn health and maternal–neonate bonding, diligent efforts by clinicians are required to achieve optimal analgesia [3,4]. Programmed intermittent epidural bolus (PIEB) infusion is a novel technique that delivers local anesthetics at programmed intervals and has gained increasing popularity as a form of labor analgesia [5–7]. Previous studies on PIEB for labor analgesia demonstrated that it reduced the incidence of motor blockade [5,6], decreased local anesthetic consumption [5], and yielded comparable or improved pain control than continuous epidural infusion (CEI) [5,6]. Furthermore, two studies comparing PIEB and CEI for post-CS analgesia reported that the PIEB also provided effective analgesia, reduced ropivacaine consumption, and decreased the need for patient-controlled epidural analgesia (PCEA) [8,9]. However, the available evidence is not sufficient to recommend PIEB for post-CS analgesia. In addition, the optimal regimen of PIEB for post-CS analgesia has not yet been established.
In this study, we aimed to compare the analgesic effects of PIEB and CEI based on pain scores at 36 h after CS. We hypothesized that PIEB would be a more effective analgesia than CEI after CS. Additionally, we compared total local anesthetic consumption, need for PCEA, and safety profiles between the PIEB and CEI groups.
Materials and Methods
This single-center, prospective, two-arm randomized controlled trial was conducted at the Samsung Medical Center in Korea between October 2022 and April 2023.
Ethics
This study protocol adheres to the Consolidated Standard of Reporting Trials (CONSORT) guidelines and the principles of the Declaration of Helsinki of 2013. The study protocol was approved by the Samsung Medical Center Institutional Review Board (SMC 2022-08-073 on September 15, 2022) and registered prospectively on the Clinical Research Information Services (KCT0007756; September 29, 2022). Informed consent was obtained from all participants prior to their enrollment in the study.
Study participants
Pregnant women scheduled for elective CS under combined spinal epidural anesthesia (CSE) were assessed for eligibility. The inclusion criteria were as follows: age between 19 and 45 years, American Society of Anesthesiologists (ASA) physical status Ⅱ, full-term pregnancy, parity with fewer than three previous births, and singleton pregnancy. Patients were excluded from the study if the body mass index was over 40 kg/m2, known fetal abnormalities were present, the study protocol could not be understood, or if participation was refused.
Randomization and blinding
Participants were randomly allocated to the CEI (control group) or PIEB groups in a 1:1 ratio. A randomization table with a block size of four was generated using a web service (www.sealedenvelope.com). Allocation information was concealed using a sealed envelope technique by independent staff not involved in the study. The anesthesia nurse sequentially opened the envelope and set up the PCEA infusion pump (Accumate 1200®, Wooyoung Medical Co., Ltd., Korea) according to group allocation in a separate room. With its monitor veiled, the infusion pump was delivered to the post-anesthesia care unit (PACU) to initiate epidural infusion. Participants, anesthesia providers, outcome assessors, and obstetricians were blinded to group assignment.
Anesthesia and postoperative management
In the operating room, CSE was performed, with participants in the lateral decubitus position, according to institutional protocols [10]. A standard sterile technique was followed. An 18-gauge Tuohy needle (Portex® epidural minipack, ICU Medical, Inc., USA) was inserted in the L2–L3 intervertebral space. The epidural spaces were identified using the loss of resistance technique with air, and a multi-orifice epidural catheter was threaded 4–5 cm into the epidural space. Following epidural catheter placement, spinal anesthesia was performed at the L3–L4 intervertebral space using a 25-gauge Whitacre needle (BD® Whitacre needle, Becton, Dickinson and Company, USA). Confirmation of free flow of cerebrospinal fluid was followed by the intrathecal administration of 8 mg of 0.5% hyperbaric bupivacaine and 100 μg of morphine sulfate to all participants. The block height for surgery was confirmed when the dermatome was desensitized up to T4 and assessed using an alcohol swab. All participants received prophylactic intravenous phenylephrine continuous infusion at a rate of 100 μg/h. Additional phenylephrine boluses (50 μg to 100 μg) were administered to maintain the mean blood pressure within 20% of the baseline value.
All participants received standardized postoperative analgesia according to the institutional protocol, with the mode of epidural infusion changed based on grouping. Epidural infusion was initiated in the PACU and maintained until postoperative day (POD) 2. Additionally, intravenous ketorolac 30 mg was administered every 8 h for 24 h after surgery; this was then switched to oral acetaminophen 650 mg every 8 h from POD 2 to POD 3. If pain control with PCEA was inadequate, intramuscular ketoprofen 100 mg was administered upon the participant’s request. Participants also received regular doses of intravenous ramosetron 0.3 mg every 8 h for POD 2.
Intervention
According to an institutional protocol, the regimen for epidural infusion consisted of a mixture of 20 ml of 1000 μg fentanyl, 40 ml of 0.75% ropivacaine, and 210 ml of 0.9% saline (i.e., total 270 ml of 0.11% of ropivacaine). An epidural infusion pump was initiated in the PACU once the first complaint of pain or resolution of spinal anesthesia to a level of T10 was achieved. Both pumps were equipped with PCEA, allowing a 2-ml bolus with a lockout time of 15 min and a maximum volume of 12 ml. The CEI group received a constant rate of 4 ml/h of 0.11% ropivacaine. In the PIEB group, the infusion pump was programmed to deliver a 4-ml bolus every hour at a rate of 120 ml/h. The first intermittent bolus through the epidural catheter was delivered immediately after connecting the epidural pump. The PIEB dosing guideline was based on our institutional experience [11]. Access to the epidural infusion regimen described above was available to all participants until POD 2; however, the infusion pump was temporarily stopped by nursing staff if the participants reported subjective motor weakness in the lower extremities. The pump was restarted once the motor weakness resolved.
Measurements and outcomes
The primary outcome of the study was the pain score at rest at 36 h after CS. Secondary outcomes included pain scores at rest and during mobilization, total volume of epidural infusion, time to first PCEA, total volume of PCEA, number of PCEA requests, incidence of motor blockade, presence of paresthesia in the lower extremity, and postoperative recovery profiles over a 36-h period after CS.
An independent outcome assessor visited each participant and assessed outcomes at a predetermined time. Pain scores at rest were assessed at 1, 8, 24, and 36 h after CS using a numeric rating scale (NRS) (0–10; 0 = no pain, 10 = worst imaginable pain). Pain scores during mobilization were assessed at 8, 24, and 36 h after CS, considering that spinal anesthesia had not fully worn off at 1 h after CS. The incidence of motor blockade and numbness or paresthesia in the lower extremities were assessed at 8, 24, and 36 h after CS. Motor blockade was defined as a score of 1 or more on the modified Bromage score that ranges from 0 to 3 (0 = able to lift legs against gravity, 1 = able to flex knee but unable to flex legs, 2 = able to move feet but unable to flex the knee, 3 = unable to move any joints) [12]. Data for epidural infusion, including the total volume of epidural infusion, time to first PCEA, total volume of PCEA, and number of PCEA requests, were automatically recorded on the epidural pump. The logs on epidural infusion were extracted to an Excel file upon study completion.
Complications related to epidural infusion, such as hypotension, nausea, pruritus, and urinary retention, were evaluated during the epidural infusion. Hypotension was defined as a reduction of 20% or more from the baseline value or a mean blood pressure of less than 65 mmHg. All participants were requested to log the time of the first ambulation, self-voiding, and flatus. Time to voiding was defined as the interval from Foley catheter removal to self-voiding. Urinary retention was defined as self-voiding failure for 6 h after Foley catheter removal. To assess post-CS recovery, participants were asked to complete the Obstetric Quality of Recovery-11, Korean version (ObsQoR-11K) questionnaires and rate their health status using a visual analog scale, 0–100 mm (0 = worst imaginable health, 100 = best imaginable health), at 36 h after CS [13]. Patient-centered outcomes, including satisfaction with postoperative analgesia and recovery, were assessed using the NRS (0 = not satisfied at all, 10 = very satisfied) at 36 h after CS.
Sample size calculation and statistical analysis
The sample size was calculated based on the results of a pilot study (unpublished data). In the pilot study, we assessed the pain scores 36 h after CS in the patient using PCEA with the CEI method. The mean pain score at rest at 36 h after CS was 4.5 ± 1.9 (mean ± standard deviation [SD]) on the NRS in the pilot study. We assumed that the pain scores at rest of the PIEB group had to decrease by 1.5 or more [14] to detect clinically meaningful differences compared with the CEI group. With an alpha error of 5% and a power of 90%, 34 participants for each group were required using two-sample t-test. Accounting for a potential dropout rate of 10%, we enrolled 37 participants in each group.
All data were tested for normality using the Shapiro–Wilk test. Normally distributed data are presented as mean ± SD and were compared using two-sample t-test. Non-normally distributed data are presented as median (Q1, Q3) and were analyzed using the Mann–Whitney U test. Categorical variables are presented as the number (%) and were compared using a chi-square test or Fisher’s exact test, as appropriate. The NRS data were analyzed using repeated-measures analysis of variance to identify the interaction between time and group for variables of multiple testing. Pain scores at each time point were compared using the Mann–Whitney U test. The time-weighted pain score was calculated as the area under the curve (AUC0–36 h) of the time–pain score curve for each participant in both groups. For post hoc analysis, the Bonferroni correction was applied in multiple comparisons of pain scores. Statistical significance was set at P < 0.05. All statistical analysis was performed using SPSS® 27.0 (IBM Inc., USA).
Results
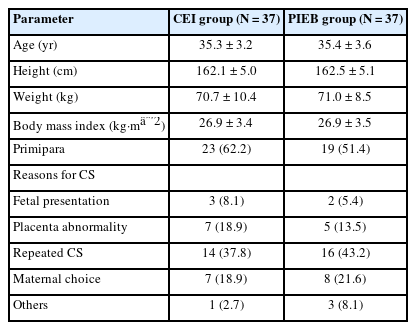
Between October 2022 and April 2023, 87 women scheduled for elective CS were assessed for eligibility. Among them, three women did not meet the inclusion criteria, and ten women refused to participate in the study. All enrolled women (n = 74) were randomly assigned to either the CEI or PIEB group and completed the intervention (Fig. 1). There were no significant differences in baseline patient characteristics between the groups (Table 1).

CONSORT flow diagram illustrating the participant enrollment and allocation during the study period. CEI: continuous epidural infusion, CONSORT: Consolidated Standard of Reporting Trials, PIEB: programmed intermittent epidural boluses.
Median pain scores (Q1, Q3) at rest measured at 8 and 24 h after CS were significantly lower in the PIEB group compared with those in the CEI group (0 [0, 1] vs. 2 [1, 3], P < 0.001; 0 [0, 2] vs. 2 [1, 3], P = 0.005, respectively; Fig. 2A). Pain score at rest at 36 h after CS, the primary outcome, was also significantly lower in the PIEB group than in the CEI group (3 [2, 4] vs. 0 [0, 2]; median difference: 2, 95% CI: 1, 2; P < 0.001; Fig. 2A). Pain scores during mobilization were significantly lower in the PIEB group than in the CEI group at all time points (8, 24, and 36 h) (Fig. 2B). Repeatedly measured pain scores at rest had a significant time–group interaction (P < 0.001). In contrast, no significant time–group interaction was observed in pain during mobilization (P = 0.079). The mean time-weighted pain score using AUC0–36 h was significantly lower in the PIEB group than in the CEI group (Pain at rest: 65.4 ± 31.4 vs. 27.9 ± 23.4, P < 0.001; Pain during mobilization: 136.3 ± 39.1 vs. 79.7 ± 33.4, P < 0.001).
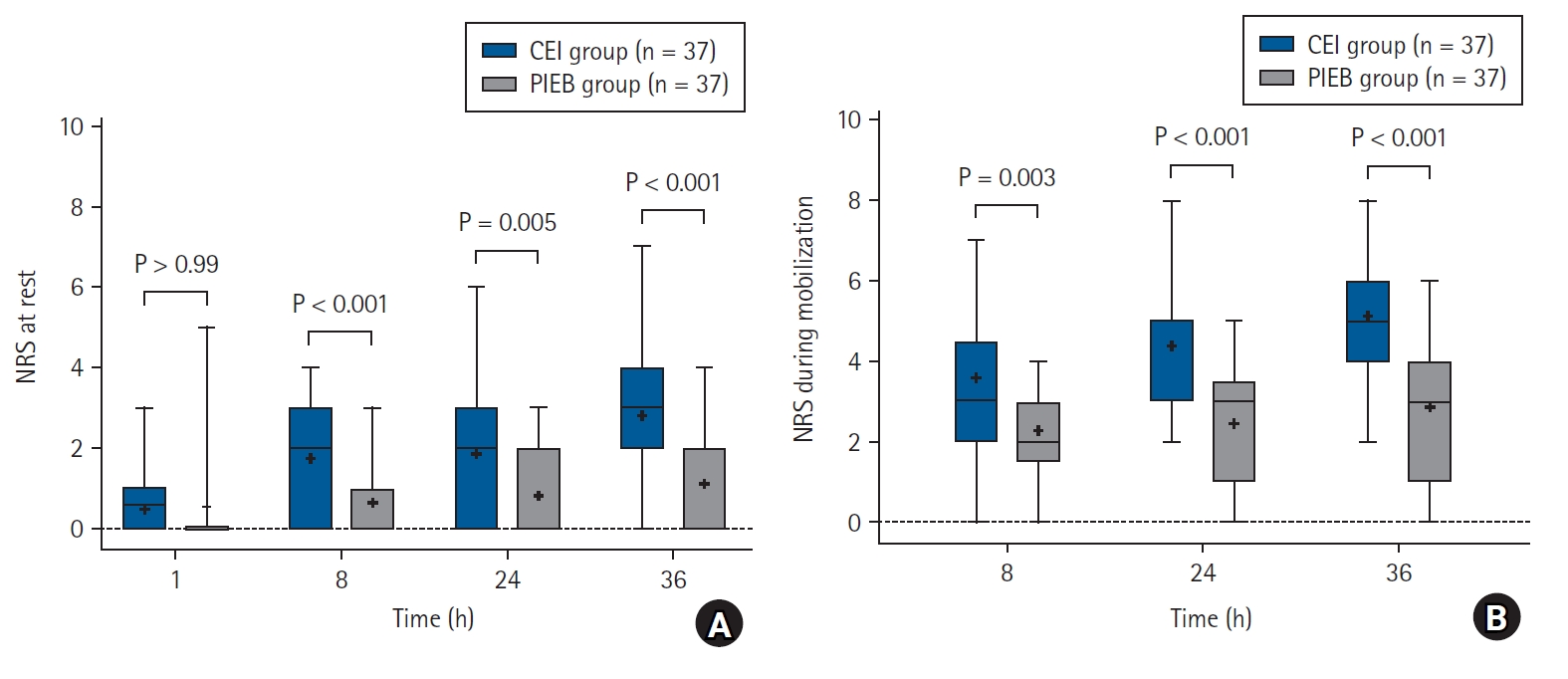
NRS scores of pain at rest (A) and during mobilization (B) during 36 h after CS. Individual P-values were adjusted using a Bonferroni correction for multiple testing, with a significance level of 0.05. CEI: continuous epidural infusion, CS: cesarean section, NRS: numeric rating scale, PIEB: programmed intermittent epidural boluses.
There were no significant differences in the total volume of epidural infusion, total volume of PCEA, and number of PCEA requests between the groups, except for the total volume of PCEA at 24 h (Table 2). The total volume of PCEA administered over a 24-h period was significantly lower in the PIEB group (2 [0, 9]) than in the CEI group (10 [4, 15], P = 0.016). The median time to the first PCEA was significantly longer in the PIEB group (9.0 [5.5, 24.2] h) than in the CEI group (5.3 [2, 10.8] h, P = 0.038).
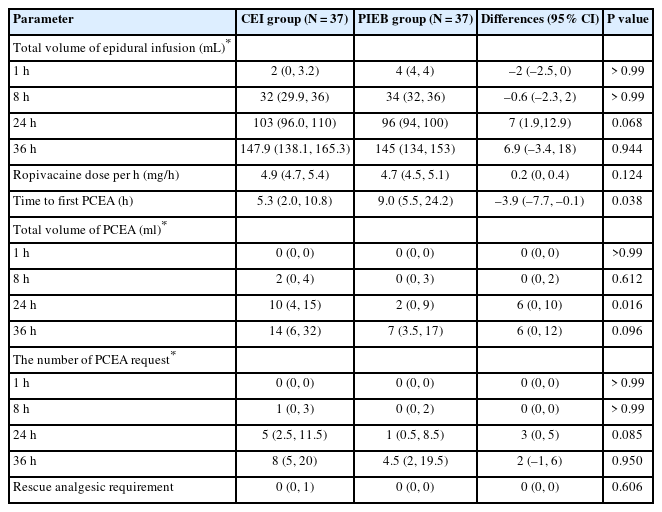
The Variables of Epidural Infusion and Rescue Analgesic Administration between the Continuous Epidural Infusion (CEI) and Programmed Intermittent Epidural Boluses (PIEB) Group
The satisfaction score for analgesia at 36 h after CS was significantly higher in the PIEB group than in the CEI group (P = 0.018; Table 3). As regards postoperative recovery profiles, participants in the PIEB group showed significantly higher ObsQoR-11K scores compared with those for participants in the CEI group (87.0 ± 15.2 vs. 73.0 ± 16.9; mean difference, –14; 95% CI, [–21.4, –6.5]; P < 0.001). Differences in each item of the ObsQoR-11K between the groups is presented in Supplementary Fig. 1.
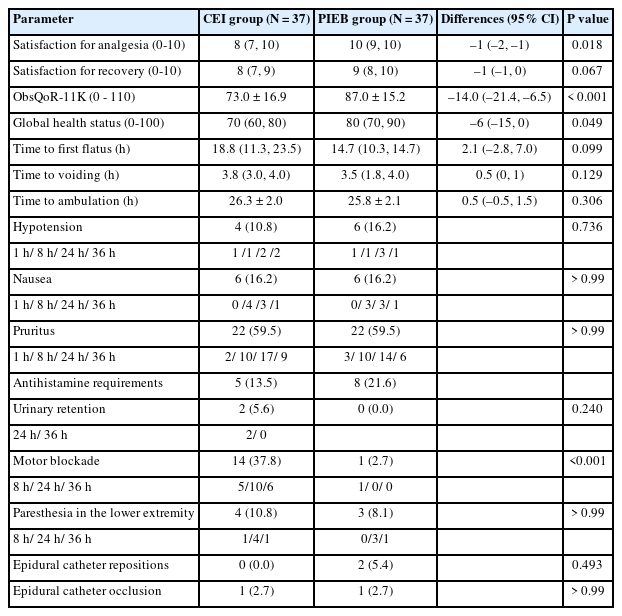
Perioperative Clinical Outcomes between the Continuous Epidural Infusion (CEI) and Programmed Intermittent Epidural Boluses (PIEB) Group
The incidence of adverse events, such as hypotension, nausea, pruritus, urinary retention, and paresthesia in the lower extremities, was not significantly different between the groups (Table 3). Notably, motor blockade, assessed within 36 h after CS, was observed in 14 participants (37.8%) in the CEI group, whereas only one participant (2.7%) in the PIEB group experienced motor blockade (P < 0.001). In the PIEB group, epidural catheter repositioning was needed in two participants (5.4%) due to unilateral block. Epidural catheter occlusion occurred in one participant (2.7%) in each group. No other adverse events were observed in both groups during the study period.
Discussion
Our findings demonstrated that PIEB provided more effective analgesia 36 h after CS than CEI without increasing the risk of motor deficits. The superior analgesic effects of PIEB were observed in pain scores at rest and during mobilization. In addition, PIEB was associated with a lower incidence of motor blockade, longer time to first PCEA, a lower volume of PCEA during the 24 h following CS, and higher maternal satisfaction with pain relief.
Our findings are consistent with those of previous studies showing that PIEB yielded more effective analgesia after CS than CEI [8,9]. Specifically, a previous study reported that PIEB using a 6-ml bolus of 0.1% ropivacaine every hour reduced the pain score of uterine contraction after CS compared to that with CEI (6 ml/h) [8]. The greater analgesic effect of PIEB over CEI can be attributed to the difference in local anesthetic spread in the epidural space. In PIEB, the higher bolus delivery rate generates a higher injection pressure of local anesthetics [15,16]. A cadaveric study demonstrated that local anesthetics tended to spread more evenly in the epidural space when administered by bolus injection compared with continuous infusion, and the spread was correspondingly uniform with high injection pressure [17]. Moreover, studies in porcine models have shown that bolus epidural injection of a dye solution produced a more extensive longitudinal and circumferential spread compared with continuous infusion, suggesting that PIEB can be more evenly distributed in the epidural space [18,19]. Consequently, the greater analgesic effect of PIEB over CEI can be attributed to the uniform spread of local anesthetics in the epidural space due to the higher injection pressure compared to CEI.
In this study, the delivery rate of PIEB (120 ml/h) was relatively low compared with that in previous studies (ranging from 250 to 360 ml/h) [5,8]. We chose a delivery rate of 120 ml/h for PIEB for several reasons. To prioritize the safety of participants, a cautious approach was adopted when configuring the epidural pump settings. A previous study at our institution demonstrated the superior analgesic effect of PIEB over CEI in labor analgesia with a delivery rate of 240 ml/h [11]. Considering the extended duration of epidural infusion and the limited availability of safety data in the CS population, we selected a reduced delivery rate of 120 ml/h, half the rate employed in the aforementioned study. In addition, a higher delivery rate was associated with occlusion of the infusion pump [16], thus prompting us to choose a relatively lower delivery rate. Finally, a previous study showed that PIEB of 100 ml/h had similar analgesia compared to PIEB of 300 ml/h [20], suggesting that a delivery rate of 120 ml/h would be sufficient to achieve effective analgesia.
The most important benefit of PIEB is that it can reduce the incidence of motor blockades [8]. Achieving the conflicting goals of ensuring adequate pain control while avoiding unnecessary sensory block and motor blockade in epidural analgesia for CS presents a major challenge. Indeed, the mechanism by which PIEB reduced the incidence of motor blockade might be attributed to differences in local anesthetic concentrations depending on the injection methods. When given in PIEB, the intraneural concentration of local anesthetics reduces as they diffuse out between bolus intervals. In contrast, the concentration of local anesthetics remains consistently higher in the extraneural spaces in the CEI, generating a concentration gradient with the intraneural space [21]. This concentration gradient causes local anesthetics to persistently move into the intraneural space, leading to motor blockade [16,21]. The lower incidence of motor blockade observed in postpartum women using PIEB in this study may be associated with the benefits of early ambulation, as recommended by the Enhanced Recovery After Surgery (ERAS) guidelines [22,23]. Early ambulation is an important clinical indicator for maternal health and neonatal care as it indicates better postoperative recovery and ensures maintenance of maternal independence during the postpartum period [24].
Another benefit of PIEB presented in previous studies is its local anesthetic-sparing effect [9,25]. Specifically, a previous study comparing PIEB and CEI for post-CS analgesia reported that PIEB (3 ml bolus of 2% ropivacaine every hour) reduced 48-h ropivacaine consumption by 20 mg compared to CEI (3 ml/h), while providing comparable analgesia [9]. However, in our study, as shown in Table 2, there was no significant difference in local anesthetic consumption between the groups. This discrepancy could be explained by methodological differences in the epidural pump start time. In this study, the epidural infusion pump was started at the same time point in both groups, and the infusion pump in the PIEB group was programmed to deliver the first intermittent bolus at the start of the epidural infusion pump. In contrast, in previous studies the first intermittent bolus in the PIEB group was administered 30 min or 1 h later compared to CEI [9,25]. A recent review article also discussed that reduced local anesthetic consumption with PIEB may result from artifacts of epidural pump start times [16]. Another possible reason is the strategy for managing epidural analgesia in our institution. The epidural infusion pump was temporarily stopped by the nursing staff if the participants reported subjective motor weakness in the lower extremities. In the CEI group, seven participants experienced subjective motor weakness, but in the PIEB group, only three participants experienced subjective motor weakness that made the epidural pump off-time longer in the CEI group. In addition, routine multimodal analgesia, including intrathecal morphine and regular acetaminophen that was administered to the patients in our study could have made a negligible difference in local anesthetic consumption.
As an exploratory finding, we noted that the ObsQoR-11K scores related to maternal independence and neonatal care (item number 7: able to mobilize independently; 8: hold baby; 9: feed/nurse baby; 10: look after personal hygiene, see online Supplementary Fig. 1) were significantly higher in the PIEB group compared with those in the CEI group. While our results suggested that a greater analgesic effect and less motor blockade with PIEB might be associated with maternal and neonatal well-being, we cannot provide conclusive evidence.
This study has several limitations. First, we included both parous and multiparous women in our study. Since postpartum pain might be different according to parity, stratified randomization by parity would be ideal to exclude baseline imbalances between groups. However, there was no statistically significant difference in parity in this study. In addition, a previous study reported no significant differences in adequate analgesia and analgesic consumption after CS according to parity [26]. Second, our participants were limited to pregnant women undergoing CS under the CSE technique. Of the neuraxial anesthesia options for CS, spinal anesthesia is preferred in many countries [27–29]. Thus, it is difficult to generalize our results to CS under other anesthetic techniques. Third, the “gold standard” in acute pain management following CS is intrathecal or epidural opioids combined with systemic analgesia, rather than the epidural infusion of local anesthetics [22,23,30]. Even when employing CSE for CS, the additional epidural infusion of local anesthetics beyond a single-shot technique is not generally encouraged due to concerns about complications, such as infection, hematoma formation, and hindering early ambulation [30,31]. However, the findings of this study suggest that the use of PIEB allows for effective pain control without the risk of motor weakness in the lower extremities, addressing the concerns about obstacles to early ambulation. Fourth, the optimal regimen (concentration, volume, interval, and delivery rate) for PIEB has not been determined. According to an institutional protocol, we used the same concentration and volume of epidural solution in PIEB as CEI (4 ml/h of 0.11% ropivacaine). Our results might differ with different anesthetic concentrations, bolus volume, interval, and delivery rates. Further studies are necessary to determine the optimal PIEB setting to maximize its analgesic effect. Lastly, in our study, the reduced incidence of the motor blockade did not shorten the time to first ambulation. The Foley catheter was removed about 20–24 h from the CSE induction time according to obstetrics protocols due to concerns of urinary retention. This might delay ambulation and mobilization in all participants and attenuated the differences in time to the first ambulation between the groups.
In conclusion, postoperative epidural analgesia with PIEB is more effective than CEI in patients undergoing elective CS under CSE. Future work is needed to determine the ideal PIEB settings to optimize its superior analgesic effect after CS.
Notes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Individual participant data and additional data are available from the corresponding author, RAK, on request indefinitely.
Author Contributions
Yu Jeong Bang (Data curation; Formal analysis; Methodology; Supervision; Writing – original draft; Writing – review & editing)
Heejoon Jeong (Conceptualization; Methodology; Project administration; Supervision; Writing – review & editing)
RyungA Kang (Conceptualization; Data curation; Formal analysis; Methodology;
Supervision; Writing – original draft; Writing – review & editing)
Ji-Hee Sung (Investigation; Writing – review & editing)
Suk-Joo Choi (Investigation; Writing – review & editing)
Soo-Young Oh (Investigation; Writing – review & editing)
Tae Soo Hahm (Data curation; Supervision; Writing – review & editing)
Young Hee Shin (Data curation; Investigation; Writing – review & editing)
Yeon Woo Jeong (Methodology; Writing – review & editing)
Soo Joo Choi (Conceptualization; Writing – review & editing)
Justin Sangwook Ko (Conceptualization; Methodology; Supervision; Writing – review & editing)
Supplementary Materials
Numeric rating scale of Obstetric Quality of Recovery-11 Korean version (ObsQoR-11K) 36 h after cesarean section. * indicates P < 0.05. ** indicates P < 0.001.