Comparison of preemptive and preventive intravenous acetaminophen on opioid consumption in pediatrics undergoing posterior spinal fusion surgery: a randomized controlled trial
Article information
Abstract
Background
Posterior spinal fusion (PSF), commonly used for adolescent idiopathic scoliosis (AIS), causes severe postoperative pain. Intravenous (IV) administration of acetaminophen has shown promise for opioid-sparing analgesia; however, its analgesic effect and optimal timing for its standard use remain unclear. Our study aimed to evaluate the analgesic effect and optimal timing of IV acetaminophen administration in pediatric and adolescent patients undergoing PSF and requiring adequate pain control.
Methods
This prospective, randomized, triple-blind trial was conducted in patients aged 11–20 undergoing PSF. Participants were randomized into three groups: the preemptive group (received IV acetaminophen 15 mg/kg after anesthetic induction/before surgical incision), the preventive group (received IV acetaminophen 15 mg/kg at the end of surgery/before skin closure), and the placebo group. The primary outcome was cumulative opioid consumption during the first 24 h postoperatively.
Results
Among the 99 enrolled patients, the mean ± standard deviation (SD) amount of opioid consumption during the postoperative 24 h was 60.66 ± 23.84, 52.23 ± 22.43, and 66.70 ± 23.01 mg in the preemptive, preventive, and placebo groups, respectively (overall P = 0.043). A post hoc analysis revealed that the preventive group had significantly lower opioid consumption than the placebo group (P = 0.013). However, no significant differences between the groups were observed for the secondary outcomes.
Conclusions
The preventive administration of scheduled IV acetaminophen reduces cumulative opioid consumption without increasing the incidence of drug-induced adverse events in pediatric and adolescent patients undergoing PSF.
Introduction
Posterior spinal fusion (PSF), a common surgical technique for correcting adolescent idiopathic scoliosis, causes severe postoperative pain due to extensive dissection, inflammation, and nerve sensitization. Managing pain in pediatric and adolescent surgical patients is challenging due to difficulties in pain assessment, concerns regarding oversedation, and worries regarding the side effects of opioids [1–3].
Acetaminophen, a widely used non-opioid drug, has a well-established safety profile and good tolerance in adolescent and pediatric patients [4]. Intravenous (IV) administration of acetaminophen offers rapid and predictable analgesic effects, particularly beneficial for all groups of patients in nil per os (NPO) status during the perioperative period [5]. However, few trials have examined the effects of IV acetaminophen in patients with PSF, and their conclusions are under debate [6,7].
Moreover, the optimal timing of IV acetaminophen administration for painful procedures in pediatric patients is not well defined. The role of acetaminophen as a preemptive analgesic, administered before surgical incision to prevent central sensitization, remains unclear [8]. Clinical trials investigating the timing of acetaminophen administration have yielded conflicting results [9,10]. Therefore, a well-designed randomized controlled study is needed to compare preemptive and preventive IV acetaminophen administration in painful procedures [11]. Our study aims to identify the optimal timing of IV acetaminophen to reduce opioid consumption in pediatric and adolescent patients undergoing PSF and requiring adequate pain control.
Materials and Methods
Ethics approval
This prospective, randomized, triple-blind trial received approval from the Institutional Review Board of Asan Medical Center (Seoul, Republic of Korea) on March 19, 2021 (IRB# 2021-0411), before patient enrollment. Written informed consent was obtained from all patients and their legal guardians after providing a sufficient explanation of the study protocol and rationale. The trial was registered at ClinicalTrial.gov (NCT04959591, Principal investigator: Won Uk Koh) and conducted following the original protocol and the Consolidated Standards of Reporting Trials (CONSORT) guidelines. We conducted this study in accordance with the Declaration of Helsinki, 2013, and followed the Consolidated Standards of Reporting Trials guidelines for study reporting.
Patient population
We enrolled adolescent and pediatric patients aged 11 to 20, classified as American Society of Anesthesiologists physical status I–III, scheduled for PSF. Trial eligibility screening was performed by one researcher, and another researcher approached the patient in the general ward for data collection. The operations were conducted by two orthopedic spine surgeons (C.S.L. and C.J.H.). Patients unable to participate due to mental impairment, developmental delay, allergies to acetaminophen or its additives, existing liver disease, dysfunction, or being deemed ineligible by the medical staff were excluded.
Participant allocation and blinding
Participants were randomized in a 1:1:1 ratio using computer-generated simple randomization in blocks of six, concealed in sealed envelopes. Each participant was assigned to one of the three groups: IV acetaminophen after anesthetic induction/before incision (preemptive group), at the end of surgery/before skin closure (preventive group), or placebo group. The principal investigator (W.U.K.), the only person with knowledge of the group allocation, performed the randomization and assigned the study drugs for each treatment arm but was not involved in pain assessment or determining the necessity of rescue analgesics. Another investigator (H.J.K.) prepared the study drugs for each patient but was blinded to the group allocations and not involved further in the study process, including clinical management, outcome assessment, and data analysis. After randomization, the subjects and their group assignments were recorded in a master log. To ensure blinding, the study drugs were concealed from all medical staff involved in the patient’s care, including anesthesiologists, orthopedic surgeons, nurses in the post-anesthesia care unit (PACU) and ward, the patients, and their guardians. Investigators and statisticians assessing outcomes were completely blind to randomization. The study drugs (acetaminophen and placebo normal saline) were prepared by the same manufacturer with identical volumes (100 ml) and bag shapes, including the port. To maintain blinding during infusion in the operating room and the ward, the drug bags were masked with non-transparent white tape and covered with a green-colored opaque envelope (Supplementary Fig. 1).
Anesthesia and analgesic protocol
The anesthetic technique was standardized, with no preoperative analgesics or sedatives administered. All patients received standard monitoring, including an arterial catheter in a radial artery and a central venous catheter in the right internal jugular vein. General anesthesia was induced with lidocaine (1 mg/kg), propofol (2–3 mg/kg), rocuronium (0.6–0.8 mg/kg), and endotracheal intubation was performed. Anesthesia was maintained with remifentanil and propofol target-controlled infusions, titrated to surgery requirements, and the depth of anesthesia was maintained at the anesthesiologist’s discretion. Depth was evaluated using a SEDLine® monitor (Masimo). Mechanical ventilation continued with a 40% O2-air mixture and end-tidal CO2 maintained at 35–40 mmHg. During the operation, somatosensory and motor-evoked potentials were monitored by a neurologist and a neurophysiologic technician. IV tramadol (1 mg/kg, maximum 50 mg) was administered before skin closure for immediate postoperative analgesia. Standardized postoperative administration of rescue analgesics was implemented. Patients were initiated on IV patient-controlled analgesia (PCA) devices (AutoMed®3200, Ace Medical) with fentanyl infusions up to a maximum of 1–1.5 μg/kg/h on demand, without a basal rate. The attending orthopedic surgeon was informed of the study drug administration that was recorded in the electronic medical record. In the PACU, if a patient’s numeric rating scale (NRS) score was four or higher, 1 μg/kg IV fentanyl was administered based on body weight.
In the general ward, patients with an NRS score ≥ 4 received tramadol (1 mg/kg or 50 mg intravenously) for breakthrough pain. For NRS scores ≥ 7, hydromorphone was administered at a dose of 0.02 mg/kg or 1 mg intravenously. Starting on the day after surgery, oral acetaminophen was routinely administered at 10 mg/kg or a maximum of 500 mg per dose, three times a day.
Study drug administration protocol
Participants in the preemptive group received 15 mg/kg (maximum 1 g) of IV acetaminophen (acetphen premix®, HK inno.N Corp.) after anesthetic induction/before surgical incision. The same dose was given postoperatively at 8-h intervals for 24 h. A single dose of placebo (0.9% Normal Saline Inj., HK inno.N Corp.) in an identical volume was given at the end of surgery before skin closure to conceal the allocation of study drugs. In the preventive group, participants received 15 mg/kg (maximum 1 g) IV acetaminophen at the end of surgery before skin closure and at 8-h intervals postoperatively for 24 h. A single dose of placebo in an identical volume was administered after anesthetic induction and before the surgical incision. The placebo group received 1.5 ml/kg of placebo (maximum 100 ml of normal saline) before surgical incision and skin closure and the same volume of normal saline at 8-h intervals postoperatively for 24 h.
Primary and secondary endpoints
The primary outcome was postoperative cumulative opioid consumption during the first 24 h after surgery, measured in IV morphine milligram equivalents (MME). Opioid consumption was recorded and converted into IV MME based on a previously published method [12].
Secondary outcomes included postoperative cumulative opioid consumption at 48 h and pain scores in the PACU at 4, 8, 24, and 48 h after surgery. The severity of postoperative pain was evaluated using an 11-point NRS (0 = no pain, 10 = worst imaginable pain). To collect NRS pain scores, patients were asked to indicate their pain levels at rest, during coughing, and at their worst. Worst and resting pain levels were defined as the most severe breakthrough pain since the last evaluation and the average resting pain, respectively. Pain during the cough was measured at the time point of the visit by requesting the patient to make a forceful cough. Additionally, the incidence of adverse drug events (such as respiratory depression, postoperative nausea and vomiting, pruritus, and constipation) was recorded. Following surgery, the length of hospital stays (from the end of surgery to discharge) and the time it took patients to ambulate, resume oral intake, and have a bowel movement were monitored and documented postoperatively. Patient satisfaction for recovery was assessed using the Korean version of the Quality of Recovery-15 (QoR-15K) questionnaire (QoR-15K: 0, very poor recovery; 150, excellent recovery) between 3 and 5 days after surgery [13]. In addition, laboratory parameters, including renal and hepatic function (creatinine, aspartate transaminase [AST], and alanine transferase [ALT]), and inflammatory markers, including C-reactive protein, were assayed preoperatively and on the day of surgery, the first, second, and fifth postoperative days.
Sample size calculation
The sample size calculation was based on the primary outcome. A retrospective review of 32 patients at our institution (15 patients in the acetaminophen group and 17 patients in the placebo group) showed that acetaminophen-treated patients had a 24-h opioid consumption of 1.84 mg/kg, while the placebo group had a consumption of 2.49 mg/kg with a standard deviation (SD) of 0.9. With 80% power and an α-level of 0.05, the required sample size was determined to be 31 patients per group using analysis of variance (ANOVA). A final sample size of 102, with 34 patients per group, was selected to facilitate a dropout rate of 10%.
Statistical analysis
All analyses were performed using R statistical software version 3.6.3® (R Foundation for Statistical Computing). A univariate statistical analysis was conducted to analyze baseline characteristics. Categorical variables were represented as numbers and percentages. Continuous variables are represented as mean ± SD or median and interquartile range, as appropriate.
For the primary outcome analysis, outcomes were compared using a one-way ANOVA for continuous data. Pairwise group comparisons for sensitivity analysis were performed using a two-sample t-test. Pairwise group comparisons compared the group that received the IV acetaminophen (including both the preemptive and preventive groups) to the placebo group. For the secondary outcome analysis, variables were analyzed using a Chi-square test, Fisher’s exact test for categorical data, one-way ANOVA, or the Kruskal–Wallis test for continuous data.
A P value of less than 0.05 was considered statistically significant. In the pairwise group comparisons, significance was based on 0.05/3 = 0.017 using the Bonferroni correction.
Results
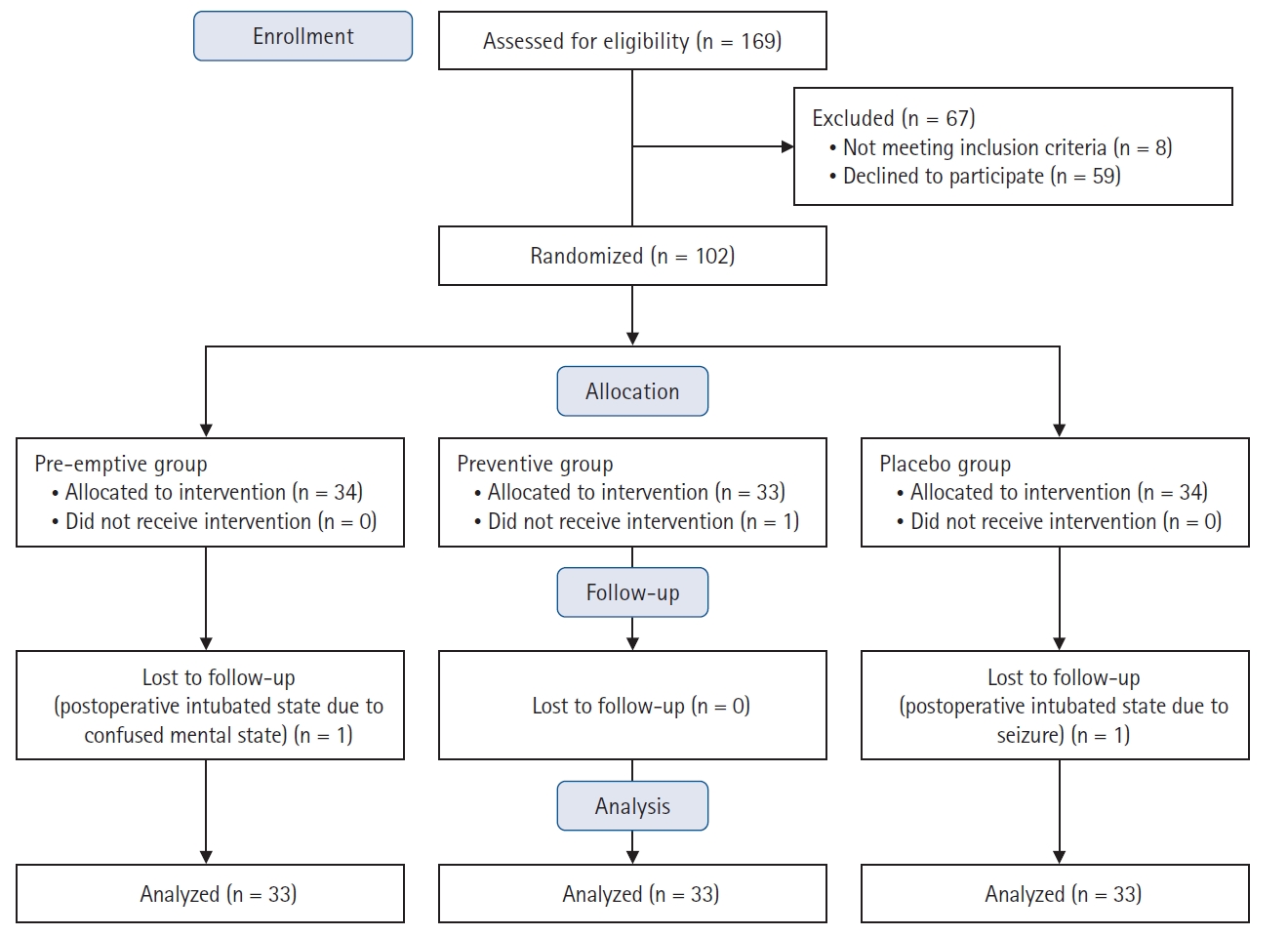
Between June 2021 and April 2022, a total of 169 pediatric and adolescent patients scheduled to undergo PSF were screened for eligibility, and 67 of them were excluded from the study (eight did not meet the inclusion criteria, and 59 declined to participate). The remaining 102 patients were assigned to one of the three groups (34 patients in each group). After excluding one patient in the preventive group due to not receiving the intervention, 101 patients were enrolled in the analysis. Two more patients were further lost to follow-up due to postoperative intubated status with sedation, leaving 33 patients in each group included in the final analysis. A CONSORT flow diagram of patient selection and dropout is displayed in Fig. 1. The baseline demographic characteristics showed no significant differences among the three groups (Table 1).
Primary outcome
The mean ± SD amount of opioid consumption during the first postoperative 24 h was 60.66 ± 23.84, 52.23 ± 22.43, and 66.70 ± 23.01 mg in the preemptive, preventive, and placebo groups, respectively (overall P = 0.043) (Fig. 2). Furthermore, the mean ± SD amount of opioid consumption per patient’s weight was 1.25 ± 0.45, 1.03 ± 0.43, and 1.34 ± 0.46 mg/kg in the preemptive, preventive, and placebo groups, respectively (overall P = 0.020). The mean differences with 95% CIs were: preemptive-placebo −0.08 (−0.30, 0.13), P = 0.443; preventive-placebo −0.30 (−0.52, 0.09), P = 0.007; and preemptive-preventive −0.22 (−0.00, 0.44), P = 0.050.
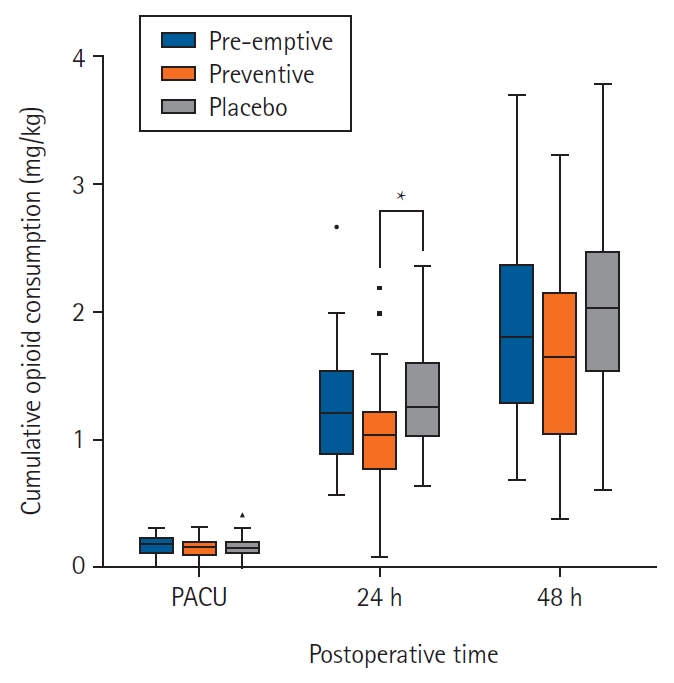
Boxplot of cumulative opioid consumption in the postoperative period. Cumulative opioid consumption was converted into IV MME. The median is represented by the central bar, the IQR by the box, and the full range by the whiskers. Outliers, defined as values over 1.5 times the IQR, are indicated by dots. *P < 0.017. IV: intravenous, MME: morphine milligram equivalent, IQR: interquartile range.
Post hoc analysis indicated significant differences in the amount of opioid consumption between the preventive and placebo groups (P = 0.013) (Fig. 2). In addition, a significant difference was observed in opioid consumption per patient’s weight between the preventive and placebo groups (P = 0.007). Total opioid consumption and total opioid consumption per patient’s weight in the preventive group compared with the preemptive group were not different (P = 0.142, 0.050, respectively). Additionally, total opioid consumption and total opioid consumption per patient’s weight in the preemptive group were not different from those in the placebo group (P = 0.291, 0.443, respectively).
In the sensitivity analysis, the mean ± SD amount of cumulative opioid consumption during postoperative 24 h in the group that received IV acetaminophen (including both the preemptive and preventive groups) was less than that in the placebo group (56.45 ± 23.35 mg vs. 66.70 ± 23.01 mg, P = 0.041) (Supplementary Table 1). The mean ± SD amount of cumulative opioid consumption per patient’s weight in the combined preemptive and preventive group was less than that in the placebo group (1.14 ± 0.45 mg/kg vs. 1.34 ± 0.46 mg/kg, P = 0.048) (Supplementary Table 1).
Secondary outcome
The total opioid consumption during postoperative 48 h was not different between the three study groups (101.36 ± 35.43 mg vs. 83.10 ± 41.47 mg vs. 90.54 ± 38.02 mg, overall P = 0.157). In addition, no significant difference was observed among the three groups in opioid consumption during postoperative 48 h divided by patient’s weight (2.03 ± 0.70 mg/kg vs. 1.62 ± 0.75 mg/kg vs. 1.86 ± 0.70 mg/kg, overall P = 0.070). No significant differences exist in resting, coughing, or the worst NRS pain scores in the PACU. Likewise, no differences were observed in resting, cough, and worst NRS pain scores at 4, 8, 24, and 48 h after surgery (Supplementary Table 2).
No difference was observed among the groups regarding the length of hospital stay (Table 2). Secondary clinical course characteristics, including time to remove the urinary catheter, first sitting time, standing time, eating time, and defecation time between the three groups, were comparable (Table 2). Compared to preoperative laboratory data, the number of patients with elevated liver function tests, including AST, ALT, and creatinine values, did not differ in the three groups (Table 2). A self-rated questionnaire for early postoperative quality of recovery evaluated by QoR-15K showed no significant difference among the study groups (Table 2). Acetaminophen treatment was well tolerated, and most of the reported adverse events were mild. The frequency of adverse events, including respiratory depression, postoperative nausea and vomiting, pruritus, and constipation, was similar among the groups. No respiratory depression or acetaminophen-induced hepatic injury that required treatment was encountered in any patient (Table 3).
Discussion
The results of this randomized controlled trial demonstrated that preventive administration of IV acetaminophen in adolescent and pediatric patients undergoing PSF reduced the cumulative 24-h opioid consumption compared to the placebo group but not in the preemptive group. This reduction in opioid consumption was 14.5 mg, exceeding the minimal clinically important difference of 10 mg reported in a previous study [14]. No significant differences were observed in pain scores, secondary clinical course characteristics, or self-reported patient satisfaction scores between the groups. The incidence of adverse events that prevented clinicians from administering IV acetaminophen to pediatric and adolescent patients did not increase compared to that in the placebo group.
Our study provides evidence of the additive effect of combining IV acetaminophen with systemic opioids, reducing opioid consumption in postoperative settings where moderate to severe pain is expected. This is consistent with previous findings that IV acetaminophen reduced opioid consumption in adult orthopedic surgery [15,16] and pediatric orthopedic surgery [17]. The different mechanisms of action of IV acetaminophen and systemic opioids, acting on different pathways and having complementary effects, may contribute to the observed additive analgesic effects. The predominant mechanisms of acetaminophen effects are central, and the most accepted theory for analgesic effects is the serotonergic descending inhibitory pathways [18,19]. However, systemic opioids exert an analgesic effect by acting on several opioid receptors in the central nervous system [20]. Therefore, these two drugs are effective as multimodal analgesia since they act on different pathways and have complementary actions. Despite this, there was no discernible disparity in the quality of pain relief as assessed by NRS scores among the three groups. This could be attributed to the effective use of PCA that allowed patients to self-administer opioids when their pain scores reached a certain threshold. The resting NRS score remained below the threshold throughout the study periods, indicating effective pain control.
The preventive administration of acetaminophen significantly reduced opioid consumption 24 h after surgery compared to the placebo group, while the preemptive administration of acetaminophen showed no difference compared to the placebo. This contradicts previous studies suggesting a preemptive analgesic effect of acetaminophen by blocking central sensitization resulting from surgical incisions [10,21]. Several reasons may be considered for these discrepancies. First, regarding the difference in the drug administration interval, the drug was administered at 8-h intervals from the end of surgery. Considering the operation time of PSF, the interval in the preemptive group was longer than 8 h. IV acetaminophen is known to have an analgesic effect within 15 min of administration, peak within 1 h, and last for 4–6 h [4,22,23]. Hence, the variation in the timing of administration between the groups likely contributed to the observed differences in the analgesic effect. Second, a potential synergistic effect may occur with the co-administration of IV acetaminophen and tramadol in the preventive group. At our institution, IV tramadol was administered when skin closure was performed to prevent remifentanil-induced hyperalgesia and to achieve a smooth awakening. Tramadol and acetaminophen do not overlap in their mechanisms of action and exhibit synergistic effects, thereby resulting in more rapid pain relief than tramadol alone and more persistent pain relief than acetaminophen alone [24,25].
The impact of reduced opioid consumption on postoperative recovery profiles was not evident in this study, indicating that the decrease in opioid usage may not be sufficient to influence postoperative recovery parameters. The adherence to established protocols for mobilization, diet, and bowel activity may have minimized differences in secondary clinical course characteristics. However, there was no difference in the incidence of adverse events among the groups, and no patients experienced hepatic injury due to IV acetaminophen exposure. According to previous studies, the estimated incidence of hepatic serious adverse events and drug-induced liver injury was only 3.2 and 0.4 per million patients, respectively [26], and most cases were related to other factors such as concomitant hepatotoxic medications, comorbid hepatic conditions, or medication errors [4]. Hence, when considering its capacity to reduce opioid usage and favorable safety profile, IV acetaminophen can be considered a valuable supplement for postoperative pain management.
A notable limitation of the current study is the relatively low dose of IV acetaminophen (45 mg/kg or 3 g per day) administered compared to the recommended dose. The recommended dose of IV acetaminophen is higher in pediatrics, with a maximum daily dose of IV acetaminophen of 60–75 mg/kg or 4 g per day [27]. The lower dose was chosen for safety concerns in the pediatric and adolescent population but may have impacted the outcomes. Future studies should investigate the relationship between drug dose and outcomes, including opioid consumption, adverse events, and recovery profiles. The second limitation is that the sample size calculation method does not take into account secondary outcomes. Nevertheless, our study was specifically structured to examine the disparity in opioid consumption with IV acetaminophen as the primary outcome, and we are confident that our sample size was adequate for this purpose.
In conclusion, the results of this study support the preventive administration of IV acetaminophen during the perioperative period in pediatric and adolescent patients undergoing a painful procedure. Moreover, it effectively reduces opioid consumption for 24 h postoperatively without increasing drug-induced adverse events and can be considered a valuable addition to postoperative pain management in this patient population.
Notes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Yeon Ju Kim (Data curation; Formal analysis; Investigation; Writing – original draft)
Ha-Jung Kim (Data curation; Formal analysis; Investigation; Methodology)
Sehee Kim (Data curation; Formal analysis)
Hyungtae Kim (Formal analysis; Methodology)
Choon Sung Lee (Methodology; Supervision)
Chang Ju Hwang (Data curation; Formal analysis)
Jae Hwan Cho (Data curation; Formal analysis)
Young-Jin Ro (Methodology; Supervision)
Won Uk Koh (Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing – original draft; Writing – review & editing)
Supplementary Materials
Blinded piggy bags containing study drugs (acetaminophen or placebo normal saline). (A) The bag on the left, marked with green letters, contains acetaminophen (1 g in 100 ml). The bag on the right, marked with blue letters, contains placebo normal saline (100 ml). (B) The study drugs were obscured by wrapping the bags with non-transparent white tape. C) To further ensure blinding, the wrapped bags were enclosed in a green-colored opaque envelope.
Cumulative opioid consumption in intravenous (IV) morphine milligram equivalents (MME) comparing the combined acetaminophen group (both preemptive and preventive groups) to the placebo group.
Postoperative pain assessment as measured by the numeric rating scale (NRS) score.