 |
 |
| Korean J Anesthesiol > Volume 77(2); 2024 > Article |
|
Abstract
Background
Esophagogastroduodenoscopy (EGD) is vital for the diagnosis and treatment of various gastrointestinal conditions but carries a low risk of venous air embolism (VAE). We report a case of VAE during EGD, confirmed by computed tomographic pulmonary angiography (CTPA).
Case
A 56-year-old male with a history of hypopharyngeal cancer underwent EGD for dysphagia-related esophageal dilation. Signs of VAE were noted, prompting swift interventions, including oxygen therapy, positional changes, and CTPA. CTPA revealed the Mercedes-Benz sign, pneumomediastinum, and a minimal pneumothorax. The patient’s oxygen saturation improved within 30 min before undergoing CTPA, and he was discharged on postoperative day 4.
Esophagogastroduodenoscopy (EGD) is a crucial procedure in gastroenterology that allows for various gastrointestinal conditions to be appropriately diagnosed, treated, and monitored. Although EGD offers invaluable information and therapeutic options, venous air embolism (VAE) is a rare but severe potential complication. As VAEs can disrupt blood flow and cause serious cardiovascular and pulmonary complications, including cardiac arrest and acute respiratory distress, clinicians must be extremely vigilant. This case report highlights the importance of promptly recognizing and managing VAE during EGD to prevent the potential adverse outcomes associated with this rare but serious complication. The aim of this case report is thus to enhance clinicians’ understanding of VAE and to emphasize the importance of its consideration during EGD procedures.
This case report obtained written informed consent from a guardian and was approved by the Human Research Ethics Committee of Faculty of Medicine, Prince of Songkla University.
A 56-year-old male patient (weight: 62 kg, height: 175 cm) who had previously undergone chemotherapy and radiation therapy for hypopharyngeal cancer developed a complete cervical esophageal stricture after undergoing external beam radiotherapy. Airway assessments revealed no limitations in mouth, jaw, or neck movement and a Mallampati score of Ⅱ. The patient underwent a scheduled esophagogastroduodenoscopy in the gastrointestinal suite four months later to dilate the esophagus and facilitate oral feeding despite the presence of a gastrostomy. The gastrointestinal suite was equipped with a pre-use checked anesthesia machine and monitor, an emergency cart, and a defibrillator.
Initially, the attending anesthesiologist administered intravenous ketamine (50 mg), midazolam (2 mg), and fentanyl (50 µg). However, because of difficulties in maintaining the airway, the attending anesthesiologist decided to switch to general anesthesia using an oroendotracheal tube. Anesthesia was maintained using sevoflurane 2.0–3.0 vol% with a mixture of 40% oxygen. Mechanical ventilation was provided at a tidal volume of 500 ml, a rate of 12 breaths/min, and a positive end-expiratory pressure of 5 mmHg. Intraprocedural monitoring included electrocardiography, noninvasive blood pressure, pulse oximetry (SpO2), and end-tidal carbon dioxide concentration (ETCO2). Air insufflation was also used during the procedure. Endoscopy revealed complete stenosis 10 cm from the incisor, with a distal site 35 cm from the gastrostomy site. The esophageal stricture was successfully dilated using the rendezvous technique.
Approximately 45 min into the endoscopy, the patient’s oxygen saturation dropped abruptly from 100% to 85% and the ETCO2 decreased from 30 to 10 mmHg. The patient had a blood pressure of 110/85 mmHg and a heart rate of 105 beats/min in normal sinus rhythm. Subcutaneous emphysema was identified in the right chest wall, while lung examinations yielded normal results. The surgical team was promptly notified and the procedure was halted. Manual administration of 100% oxygen was initiated and a 500-ml bolus of normal saline was administered to maintain preload. The patient’s position was then changed to Trendelenburg. While waiting to undergo computed tomographic pulmonary angiography (CTPA) after 30 minutes of attempted resuscitation, the patient had a blood pressure of 130/80 mmHg, oxygen saturation of 95%, heart rate of 80 beats/min, and ETCO2 of 28 mmHg.
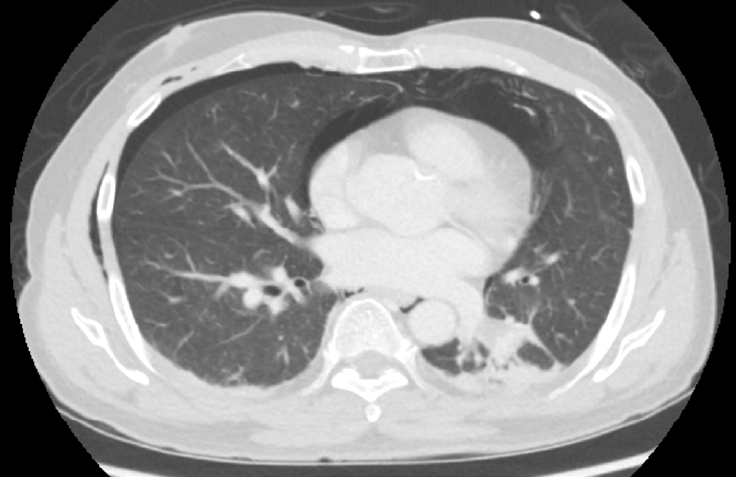
CTPA was conducted to rule out a pulmonary embolism while an adequate depth of anesthesia was maintained using midazolam and sevoflurane at a minimum alveolar anesthetic concentration (MAC) of 0.5. CTPA results revealed minimal air bubbles in the pulmonary trunk, forming a tri-radiate offshoot artifact (dynamic Mercedes-Benz sign) attributed to pulsation (Fig. 1) [1]. Diffuse pneumomediastinum and minimal right pneumothorax were also observed (Fig. 2). Mechanical ventilation was continued until postoperative day 2, and the patient was discharged on postoperative day 4.
Endoscopic procedures often require sedation. Adverse events experienced under non-operating room anesthesia are similar to those experienced in the operating room, but the frequency and implications differ. Anesthesiologists encounter challenges in unfamiliar environments, such as limited space, confusion regarding the location of critical equipment and supplies, and restricted access to both the patient and airway. Ensuring patient safety is pivotal for anesthesiologists, encompassing appropriate collaboration with the team, management of a mobile anesthesia cart adequately equipped for emergencies, and the implementation of an effective system of communication during emergencies. Adequate anesthesia manpower is also crucial for patient safety. Anesthesiologists that practice regularly have been shown to be more efficient than non-regular anesthesiologists, as demonstrated by higher mean oxygen saturation, lower operating room turnaround time, and lower operating room costs [2].
EGD is a versatile procedure with diagnostic and therapeutic applications including the management of complex conditions (e.g., esophageal strictures) that often involve the rendezvous technique. Although complications associated with esophageal dilation are relatively rare, they can include esophageal or hypopharyngeal perforation, abdominal wall infection, stomach wall dehiscence, and pneumothorax [3]. Although rare, VAE is a severe and potentially fatal complication that is primarily iatrogenic and has been documented in various medical procedures, including sitting craniotomy, pars plana vitrectomy, cesarean delivery, and gastrointestinal procedures such as endoscopic retrograde cholangiopancreatography and EGD [4].
Clinical manifestations of VAE are diverse and can involve both the cardiovascular and neurological systems. However, in certain instances, the effects may be obscured by anesthesia, making prompt recognition difficult. The severity of VAE is closely linked to the rate and volume of air introduced into the circulatory system. Smaller volumes may cause subclinical effects, whereas larger volumes can significantly disturb a patient’s hemodynamics. The symptoms of VAE are nonspecific, meaning that even the smallest suspicion should prompt a thorough investigation and diagnosis. Several risk factors are associated with VAE, including inflammatory conditions of the bile duct, hepatic abscesses, inflammatory bowel diseases, necrotizing enterocolitis, and gastrointestinal tumors. Procedural factors that can increase the risk of VAE include air insufflation and the use of nitrous oxide [5]. Most cases of VAE occur in conjunction with a disruption in the mucosal barrier, such as an ulceration, dilation, biopsy, or sphincterotomy.
In this patient, VAE likely occurred due to direct air entry into the exposed blood vessels during esophageal stricture dilation, resulting in a connection between the esophageal lumen and venous circulation [6,7]. Additionally, the use of high-pressure air insufflation during the procedure may have facilitated air entry [8]. Notably, the insufflation pressure was not continuously monitored. Furthermore, exposure of the endoscope to radiation may have resulted in inflammatory changes, predisposing the esophagus to VAE [3]. A rapid reduction in ETCO2 is a highly sensitive indicator of VAE at a threshold of 0.25 ml/kg of air and can be used to identify VAE in patients without hemodynamic compromise [9]. In cases of suspected VAE, a pulmonary embolism must be excluded, particularly in patients with underlying cancers. Therefore, the use of diagnostic tools, such as CTPA, is recommended. CTPA exhibits high sensitivity (83%) and specificity (96%) for detecting pulmonary embolisms [10]. Despite immediate bowel decompression and initial management with 100% oxygen, hemodynamic maintenance, and Trendelenburg positioning, air persisted in the pulmonary artery for an hour. The time to resolution of symptomatic air embolisms can vary from 5 to 12 h [11,12]. The rate of air resorption may depend on the initial size and volume of the pulmonary embolism. The precise volume of air that can cause hemodynamic disturbances is uncertain; however, even small amounts (e.g., 50–100 ml) can be fatal in humans [12]. VAE can also be diagnosed with point-of-care ultrasonography (POCUS) using transesophageal echocardiography (TEE), which can be conducted at the bedside and provides information on signs of cardiopulmonary compromise [13]. However, the volume of air required for the detection of VAE by TEE is larger than that for CTPA, and the sensitivity may vary depending on the operator’s experience and interpretive errors [13]. Thus, small symptomatic pulmonary air embolisms may be missed on TEE.
Our CTPA findings showed minimal air bubbles in the pulmonary trunk as a tri-radiate offshoot artifact (dynamic Mercedes-Benz sign) in the upper section of the anatomic structures. This differs from respiratory artifacts, which have a distinct appearance in the middle or lower sections of vessels and typically appear as lines or other long shapes [14]. To optimize diagnosis, we adjusted the images to a relatively high window width (400–1600 HU) and a window center at approximately 40 HU with a lung window setting ranging from 1200 to −400 HU [15]. These settings can be used to clearly distinguish air bubbles representing a tri-radiate offshoot artifact from other artifacts.
Anesthesiologists play a crucial role in the prevention and management of VAE during endoscopic procedures. Prevention of VAE is paramount and can be effectively addressed using prophylactic measures including the identification of high-risk patients, maintenance of adequate venous pressure, and vigilant monitoring for signs of VAE. Continuous monitoring of vital signs, ETCO2 levels, and central venous pressure coupled with awareness of the patient’s medical history can facilitate the early detection of VAE. In terms of procedural factors, opting for carbon dioxide (CO2) insufflation over air insufflation is a viable strategy for eliminating the risk of VAE. CO2 has a significantly greater solubility than air (by approximately 50 times). This increased solubility widens the safety margin for inadvertent entry of gas into the circulatory system [4]. However, procedures during EGD may be limited when the Trendelenburg position or a posture that elevates the patient’s upper body above heart level is used.
In summary, EGD is a valuable procedure with various diagnostic and therapeutic applications, including complex cases such as esophageal strictures. However, the infrequent occurrence of VAE underscores the importance of vigilant monitoring, early detection, and prompt intervention to ensure patient safety. CTPA plays a pivotal role in the diagnosis and management of VAE, particularly in patients with underlying cancers.
NOTES
Fig. 1.
(A) Axial and (B) coronal CT pulmonary angiography (CTPA) images showing minimal air bubbles in the pulmonary trunk (arrow), indicating a tri-radiate offshoot artifact (dynamic Mercedes-Benz sign) due to pulsation.
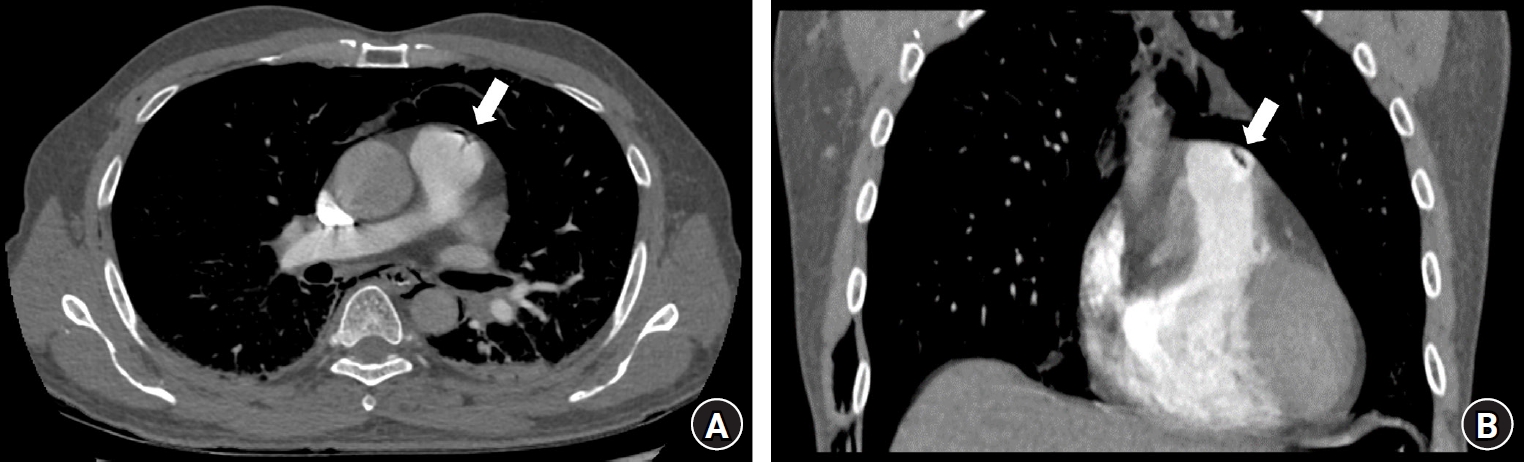
Fig. 2.
Axial CT image using lung window setting showing diffuse pneumomediastinum and minimal right pneumothorax.
References
1. Emby DJ, Ho K. Air embolus revisited - a diagnostic and interventional radiological perspective (bubble trouble and the dynamic Mercedes Benz sign). SA J Radiol 2006; 10: 3-7.


2. Goudra BG, Singh PM, Sinha AC. Anesthesia for ERCP: impact of anesthesiologist’s experience on outcome and Cost. Anesthesiol Res Pract 2013; 2013: 570518.




3. Zald PB, Andersen PE. Fatal central venous air embolism: a rare complication of esophageal dilation by rendezvous. Head Neck 2011; 33: 441-4.


4. Prielipp RC, Brull SJ. Vascular air embolism and endoscopy: every bubble matters. Anesth Analg 2018; 127: 333-5.


5. Donepudi S, Chavalitdhamrong D, Pu L, Draganov PV. Air embolism complicating gastrointestinal endoscopy: a systematic review. World J Gastrointest Endosc 2013; 5: 359-65.



6. Green BT, Tendler DA. Cerebral air embolism during upper endoscopy: case report and review. Gastrointest Endosc 2005; 61: 620-3.


7. Mittnacht AJ, Sampson I, Bauer J, Reich DL. Air embolism during sigmoidoscopy confirmed by transesophageal echocardiography. J Cardiothorac Vasc Anesth 2006; 20: 387-9.


8. Shah R, Shah S. Diffuse bi-hemispheric cortical infarction secondary to cerebral air embolism after elective esophagogastroduodenoscopy: a case report and review of literature. Cureus 2023; 15: e36069.



9. Kim CS, Liu J, Kwon JY, Shin SK, Kim KJ. Venous air embolism during surgery, especially cesarean delivery. J Korean Med Sci 2008; 23: 753-61.



10. Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006; 354: 2317-27.


11. Buckridge N, Frisch S, Sinert R. Iatrogenic pulmonary air embolism with rapid resolution: a case report. J Emerg Med 2021; 61: 172-3.


12. Jhamnani S, Panza JA. Power injector associated iatrogenic air embolism to the right heart. Thorax 2014; 69: 500.


13. Park YH, Kim HJ, Kim JT, Kim HS, Kim CS, Kim SD. Prolonged paradoxical air embolism during intraoperative intestinal endoscopy confirmed by transesophageal echocardiography -a case report-. Korean J Anesthesiol 2010; 58: 560-4.












