Introduction
Patients with end-stage liver disease frequently have cardiovascular dysfunction, termed cirrhotic cardiomyopathy [
1] that is characterized by an impaired contractile response to stress. The cause of cirrhotic cardiomyopathy is unclear; however, this syndrome is considered to be related to both portal hypertension and cirrhosis [
2]. Advanced liver disease is associated with a hyperdynamic circulatory state characterized by high cardiac output and low systemic vascular resistance [
3]. Furthermore, arterial compliance is directly related to cirrhosis severity and hyperdynamic circulatory derangement. Although liver transplantation (LT) is known to reverse cardiovascular dysfunction within 6–12 months [
4], progression of pre-existing or new-onset cardiac events has been reported [
5], possibly due to acute changes in loading conditions in the immediate postoperative period.
The left ventricular (LV) pressure–volume relationship, estimated from echocardiographic measurements, has been proposed to assess the interaction between the arterial and ventricular systems [
6]. Ventriculoarterial coupling (VAC) is defined as the ratio of arterial elastance (E
a) to LV end-systolic elastance (E
es). Suga [
6] first proposed the use of VAC to evaluate interactions between cardiac performance and vascular function. Although the interaction of the left ventricle (LV) with the arterial system, or VAC, is a key determinant of cardiovascular performance [
7], little is known about the changes in E
a, E
es (stiffness), and VAC (E
a/E
es) after LT. Therefore, we evaluated the E
a, E
es, and E
a/E
es ratios in patients undergoing LT, as well as alterations in these parameters immediately after LT. Furthermore, we examined whether decoupling the ventriculoarterial relationship was associated with postoperative complications and length of hospital stay.
Results
None of the patients received inotropic agents or vasopressors preoperatively. The baseline characteristics of the 240 patients are summarized in
Table 1.
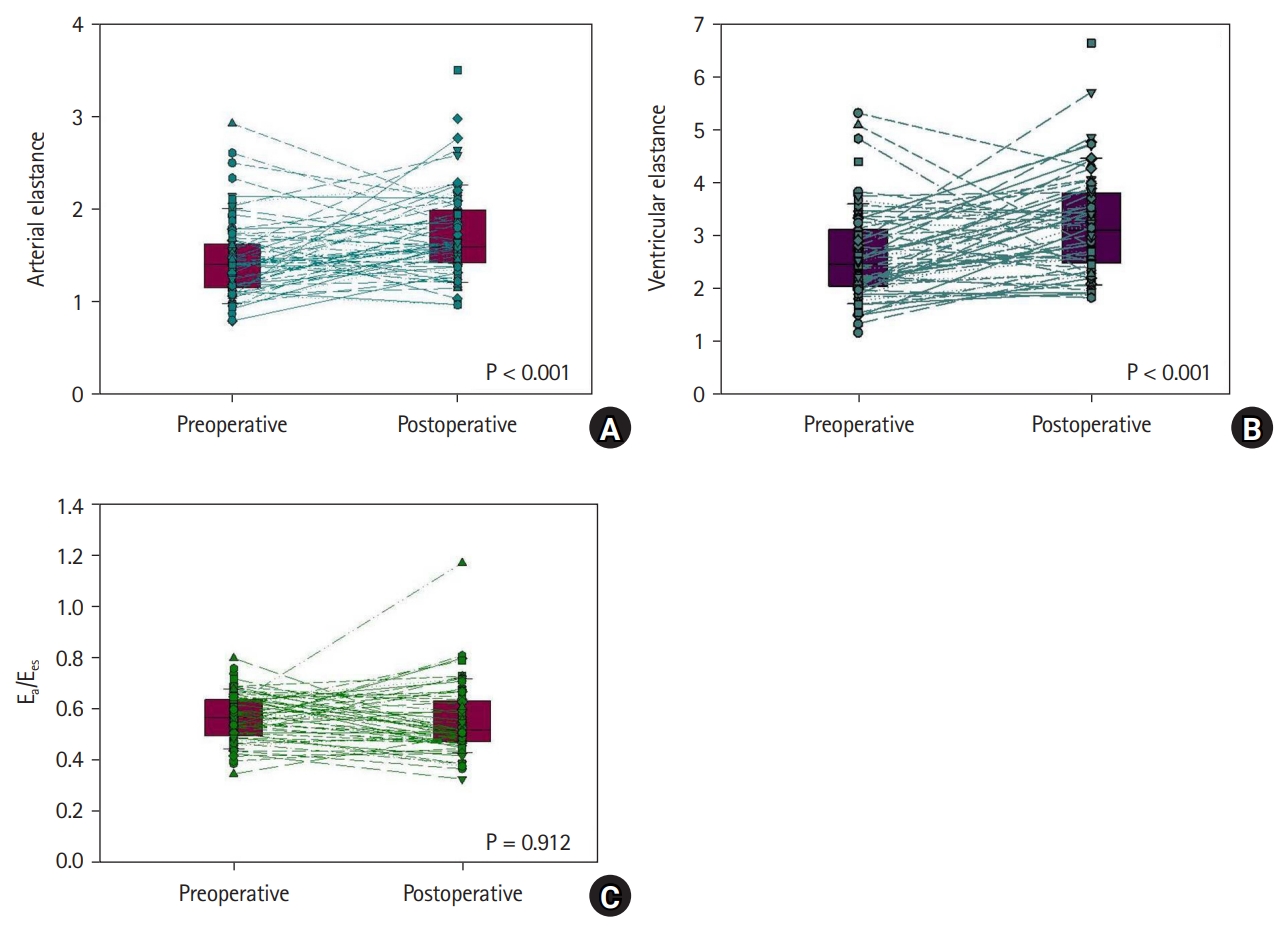
All patients underwent echocardiography within 30 postoperative days (16 [14, 19]) before discharge from the hospital. After LT, the ESP was elevated from 98 ± 12 to 107 ± 14 (P < 0.001) and was associated with an increase in LV mass index. Cardiac volume decreased, as evidenced by left atrial volume (P < 0.001), LV ESV (P = 0.010), LV EDV (P = 0.001), and SV (P = 0.001) (
Table 2).
E
a was highly correlated with the SV/PP ratio (preoperative and postoperative, r = −0.799 and −0.738, respectively). Thirty days following LT, E
a increased by 16% (P < 0.001) and TAC decreased by 14% (P < 0.001). Additionally, E
es was correlated with the LV ejection fraction (EF) and S' preoperatively (P = 0.032 and P < 0.001, respectively) and postoperatively (P = 0.048 and P < 0.001, respectively). E
es and the contractility index of S' increased by 18% (P < 0.001) and 7% (P < 0.001), respectively; however, LV EF was unaltered (P = 0.427). Given the increase in E
a and E
es, the E
a/E
es ratio remained unchanged (0.56 to 0.56, P = 0.912) (
Fig. 1). LV diastolic function changed immediately after LT within one month and the E/A ratio decreased by 16% (P < 0.001), whereas E/E' and E
ed increased by 5% (P = 0.017) and 6% (P < 0.001), respectively, in association with an increase in LVEDP (P = 0.017).
The median lengths of postoperative ICU and hospital stays were 2 days (1, 3) and 22 days (20, 29), respectively (
Table 3). In the univariate linear regression analysis, the postoperative hospital stay length was significantly associated with the Model for End-Stage Liver Disease (MELD) score (r = 0.283, P < 0.001), postoperative E
a (r = 0.157, P = 0.016), E
es (r = -0.157, P = 0.019), and E
a/E
es ratio (r = 0.695, P < 0.001). However, the postoperative E/A ratio (P = 0.967), E/E' (P = 0.939), and S' (P = 0.536) were not significantly correlated with the postoperative length of stay (
Table 4). Patients with prolonged postoperative hospital stays (≥ 42 days; 90th percentile) had a higher postoperative E
a/E
es ratio (0.54 ± 0.10 vs. 0.71 ± 0.47, P = 0.005), E/E' (9.7 ± 2.6 vs. 11.5 ± 4.4, P = 0.006), and LVEDP (17.7 ± 1.5 vs. 18.8 ± 2.6, P = 0.006), and lower LV EF (65.0 ± 4.6 vs. 61.3 ± 8.5, P = 0.003) and S' (9.2 ± 1.7 vs. 8.4 ±2.1, P = 0.032) (
Table 5). On multivariate regression analysis, high MELD score (odds ratio [OR]: 1.126, 95% CI [1.073, 1.182], P < 0.001) and higher postoperative E
a/E
es ratio (OR: 1.467; 95% CI [1.010, 2.049], P = 0.038) were identified as independent risk factors for longer postoperative hospital stays (
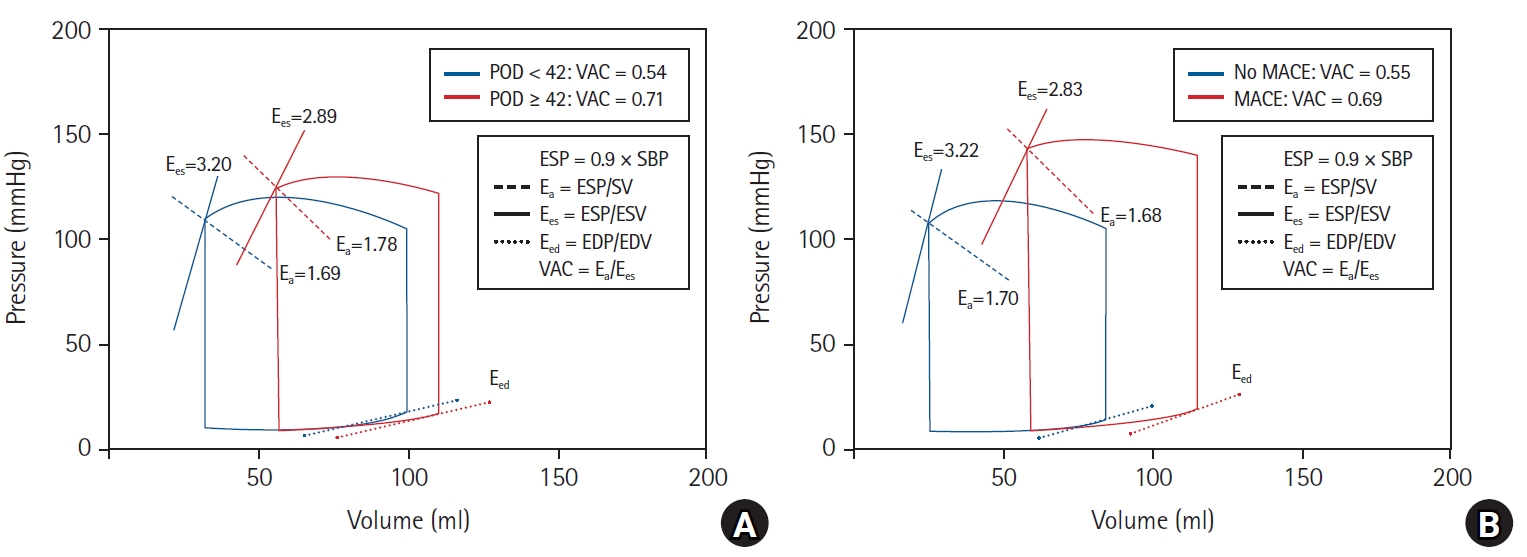
Table 6). In patients with a prolonged hospital stay after LT, an increase in E
a without an increase in E
es led to a higher VAC; however, this was not observed in patients who were discharged within 42 days postoperatively (
Fig. 2A).
Of these patients, 29 (12.1%) had MACE before hospital discharge (
Table 3). MACE was significantly associated with the MELD score (P = 0.005), postoperative ESV (P = 0.002), EDV (P = 0.017), LV EF (P = 0.018), E/E' (P = 0.009), LVEDP (P = 0.009), and E
a/E
es ratio (P = 0.008). Compared with patients without postoperative MACE, patients with MACE had decreased E
es, whereas E
a was similar; therefore, E
a/E
es ratio increased, and the pressure–volume loop shifted to the right side (
Fig. 2B). Patients with MACE had longer postoperative hospital stays (25.77 ± 14.32 days vs. 67.14 ± 97.11 days, P = 0.030). Multivariate regression analysis identified high MELD score (OR: 1.064, 95% CI [1.017, 1.113], P = 0.008), lower LV EF (OR: 1.514; 95% CI [1.079, 2.123], P = 0.016), and higher postoperative E
a/E
es ratio (OR: 6.347, 95% CI [1.672, 24.091], P = 0.007) as independent risk factors for postoperative MACE (
Table 6).
Discussion
This study investigated the characteristics of the pressure–volume relationship in patients undergoing LT and alterations in these parameters immediately after LT. We found that despite elevations in the arterial load in the 30 days following LT, VAC remained unaltered in normally discharged patients without MACE. However, patients with postoperative MACE had a significantly higher E
a/E
es ratio than those without MACE (
Fig. 2). Additionally, the development of MACE was associated with prolonged postoperative hospital stay that was significantly correlated with a higher postoperative E
a/E
es ratio. Our findings suggest that in patients discharged within one month after LT, LV contractility increases and the resulting increase in arterial load leads to the maintenance of VAC. However, if E
es did not increase more than the changes in E
a, the ratio of E
a to E
es increased and ventriculoarterial decoupling developed.
We analyzed the LV pressure–volume curve to assess the net interaction of the ventriculoarterial system. E
es is a load-independent index of myocardial contractility and LV inotropic efficiency [
12]. Similarly, in our study, preoperative and postoperative E
es correlated with other measures of LV contractility, such as EF and S'. Although LV systolic performance can be significantly influenced by the structural and geometric characteristics of the LV, it is mainly derived from myocardial contractility [
13]. Collectively, these results suggest that E
es is reflective of LV systolic function. On the ESP–volume line, E
a is quantified by the SV ejected from the ventricle against the arterial load that should be overcome by the LV. Compliance is elevated in patients with end-stage liver disease, and the SV/PP ratio reflects abnormalities in arterial compliance in these patients [
10]. In addition, E
a is inversely correlated with TAC [
14]. Our results showed that the SV/PP ratio was highly inversely correlated with E
a, a measure of net arterial load, and may represent TAC in patients undergoing LT. Torregrosa et al. [
4] found that cardiac alterations in cirrhosis were reversed within 6–12 months after LT by normalization of the systolic response. In addition, some studies have reported that TAC increased within 2–6 months after transplantation [
15,
16]. To our knowledge, this reversibility is generally observed within 30 days after transplantation, and LV systolic function increases concordantly with arterial stiffness, thereby maintaining VAC. This study is the first to analyze changes in arterial and ventricular elastance within one month of LT.
In the present study, the pressure–volume characteristics of patients with poor postoperative outcomes were fairly different from those of normally discharged patients without complications. Although studies have reported conflicting results, increases in the ventricular chamber size and volume have also been reported in patients with liver cirrhosis [
17,
18]. Our study found that the left ventricular ESV, EDV, and SV in patients with poor postoperative outcomes were higher than those in patients without complications. Alterations in LV volume and pressure are expected to contribute to the pressure–volume curve [
19,
20]. Consequently, a decrease in E
es and an increase in ESP, ESV, and EDV induced a pressure–volume curve shift to the right in patients who developed MACE and had longer postoperative hospital stays (
Fig. 2). We also observed that VAC increased in patients with poor outcomes. This suggests that within one month after LT, the LV systolic function increased and matched the arterial load in normally discharged patients without complications; however, a relative increase in VAC adversely affected the clinical outcome. The E
a/E
es ratio is an important determinant of the net cardiac performance [
21]. Contractility or arterial tone that is too high or too low decouples these processes and can lead to cardiac failure independent of myocardial ischemia or toxic effects. Both cardiac and arterial decoupling can lead to acute hemodynamic decompensation that is classified according to the underlying pathophysiological mechanisms. Because acute hemodynamic impairment should be treated based on the etiological mechanism of cardiovascular dysfunction, it is very important to evaluate the VAC. Although alterations in the VAC and its components, E
a and E
es, have been reported in aging, hypertension, and heart failure [
22], data on patients with end-stage liver disease are lacking. This was the first study to examine changes in arterial and LV elastance after LT. According to our results, MACE within one month of LT seemed to develop due to ventriculoarterial decoupling because LV contractility did not increase in accordance with the arterial load. Additionally, these patients had longer postoperative hospital stays. In patients with cirrhotic cardiomyopathy, decoupling of the ventriculoarterial interaction after surgery can occur because of a reduction in the cardiac reserve. Collectively, these results suggest that perioperative assessment of the estimated VAC derived from echocardiography may be a valuable indicator of postoperative cardiovascular dysfunction and may help guide therapeutic strategies.
This study had some limitations. First, this was a retrospective study conducted at a single medical center. Therefore, the timing of the BP measurements and echocardiography was not controlled. Second, we did not measure E
a and E
es from the pressure–volume loops acquired during cardiac catheterization. However, this technique is invasive and its use in humans is limited; therefore, non-invasive assessments of E
a and E
es have been developed in previous studies [
23,
24]. Non-invasively obtained E
a/E
es ratios have been shown to closely approximate those obtained invasively [
25]. Further prospective studies are needed to validate the gold-standard invasive methods. Third, E
es estimated by non-invasive single-beat determination using echocardiography when V
0 was designated as a volume axis intercept was considered reliable [
6]. However, we simply regarded the ratio of ESP to ESV as E
es, assuming that V
0 was negligible compared with ESV [
7]. If V
0 had been used in the calculations, the results would have differed. Further studies are needed to examine the pressure–volume relationship using V
0.
In conclusion, despite an elevated arterial load within 30 days after LT, VAC remained unaltered in patients discharged without MACE. Myocardial stiffness and contractility were increased to match the arterial load in patients without MACE. However, patients with postoperative MACE had significantly higher Ea/Ees ratios than those without MACE. Our results suggest that MACE within one month postoperatively seemed to develop due to ventriculoarterial decoupling because the LV contractility did not increase and match the arterial load. Therefore, perioperative assessment of the Ea/Ees ratio derived from echocardiography may be valuable for predicting postoperative cardiovascular dysfunction and guiding therapeutic strategies.