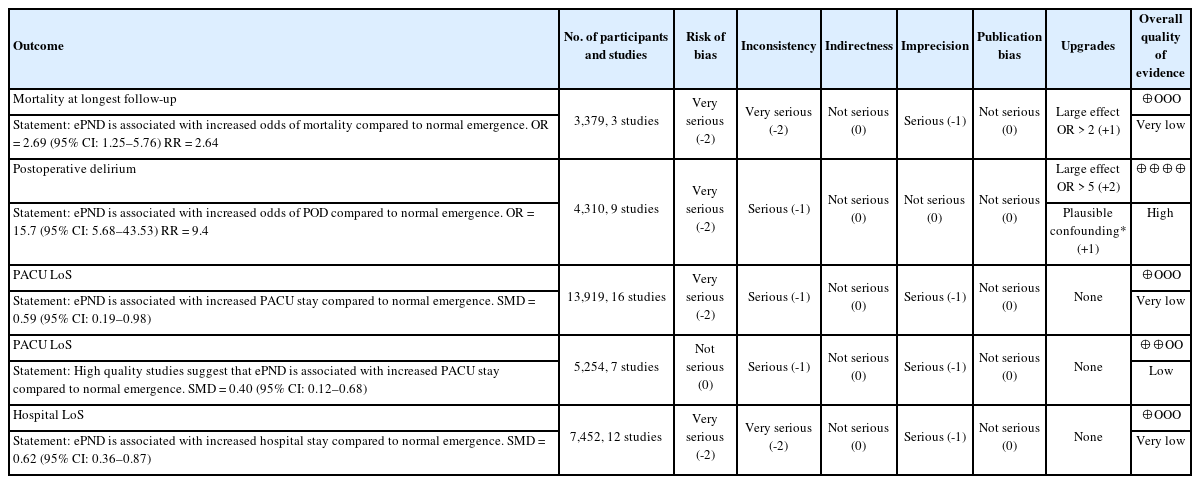
Effects of early postoperative neurocognitive disorders on clinically relevant outcomes: a meta-analysis
Article information
Abstract
Background
Early postoperative neurocognitive disorders (ePND), include both emergence delirium, which is defined as very early onset postoperative delirium, and emergence agitation, defined as motor arousal. Although research on anesthesia emergence is limited, ePND are likely associated with unfavorable outcomes. This meta-analysis assessed the effect of ePND on clinically relevant outcomes.
Methods
A systematic search of studies published between 2002 and 2022 on MEDLINE, PubMed, Google Scholar, and the Cochrane Library was performed. Studies that included adults with emergence agitation and/or delirium and reported at least one of the following outcomes: mortality, postoperative delirium, length of post-anesthesia care unit stay, or length of hospital stay were included. The internal validity, risk of bias, and certainty of the evidence were assessed.
Results
A total of 16,028 patients from 21 prospective observational studies and one case-control retrospective study were included in this meta-analysis. The occurrence rate of ePND was 13% (data excluding the case-control study). The mortality rate was 2.4% in patients with ePND vs. 1.2% in the normal emergence group (risk ratio [RR]: 2.6, P = 0.01, very low quality of evidence). Postoperative delirium occurred in 29% of patients with ePND and 4.5% of patients with normal emergence (RR: 9.5, P < 0.001, I2 = 93%). Patients with ePND had a prolonged length of post-anesthesia care unit stay (P = 0.004) and length of hospital stay (P < 0.001).
Conclusions
This meta-analysis suggests that ePND are associated with twice the risk of mortality and a 9-fold increased risk of postoperative delirium.
Introduction
Every year, more than 300 million patients undergo surgeries worldwide [1]. Postoperative complications are a major public health issue that have a negative impact on survival, quality of life, and economic costs [2–4].
Neurological disorders (e.g., delirium and cognitive decline) are common postoperative complications and are associated with increased mortality and morbidity in various populations [5]. Although no consensus on the classification of postoperative neurological disorders currently exist [6,7], experts recommend they be divided into short-term (postoperative delirium) and long-term (postoperative cognitive disorders) disorders [7].
Early postoperative neurocognitive disorders (ePND) occur immediately after surgery and consist of two entities: emergence agitation [6] and emergence delirium [6,7]. Agitation is a well-known condition [8] defined as ‘motor arousal’ that is manifested by purposeless behaviors such as fidgeting, shifting, fiddling, inability to sit or stand still, and wringing of the hands [9]. Emergence delirium, on the other hand, has more recently been assessed in the literature and is defined as very early onset postoperative neurological disorders (e.g., immediately after anesthesia, before or on arrival in the recovery room) [6]. Although the negative effects of short- and long-term postoperative neurological disorders are well established, no comprehensive data on the effect of ePND on mortality and other clinically relevant outcomes are currently available.
This study aimed to evaluate the hypothesis that patients with ePND have an increased risk of mortality and morbidity compared to those with normal emergence from anesthesia.
Materials and Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD 42022382008).
Eligibility criteria
The inclusion criteria for this study were as follows: prospective or retrospective studies on adults with emergence agitation and/or emergence delirium that reported at least one of the following outcomes: all-cause mortality, postoperative delirium, post anesthesia care unit (PACU) length of stay, and overall hospital length of stay.
We excluded studies that fulfilled at least one of the following criteria: 1) letters without patient data, 2) case reports, 3) reviews, 4) meta-analyses, 5) pediatric patients, 6) interventional studies, and 7) non-English language articles.
Search strategy
A systematic literature search of MEDLINE, PubMed, Google Scholar, and the Cochrane Library for studies published between 2002 and 2022 (total of 20 years) was performed by three independent researchers (LB, NE, and MY). Medical Subject Heading (MeSH) terms were used. Details regarding the queries are available in the supplemental material (Supplementary Material 1). Additionally, the backward snowballing method (analysis of the references of the included articles and retrieved reviews) was used to retrieve further studies.
Study selection
After removing duplicates, the titles and abstracts of the remaining studies were independently screened by two researchers. Prospective and retrospective observational studies comparing postoperative outcomes of patients with ePND to those with normal emergence were considered.
During emergence after anesthesia, patients with ePND exhibit signs of either agitation or delirium. Agitation was defined as motor arousal according to the Richmond Agitation-Sedation Scale or a similar condition according to other motor scales matching the definition ‘agitation’ in the International Classification of Diseases (ICD)-11 [9] or Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [11]. Delirium was defined using the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) [12] or the Nursing-Delirium Screening Scale (Nu-DESC) [13] and/or DSM-5 criteria [11].
The inclusion and exclusion criteria were applied to the full texts of potentially eligible studies by LB, NE, and MY. Disagreements were resolved by consensus with the involvement of the supervisor.
Outcome measures and data extraction
Data were extracted by two independent authors (LB and MY). These data included the first author, year of publication, study design, ePND criteria, sample size, mean age, sex, American Society of Anesthesiologists (ASA) status, method of anesthesia administration, operation and anesthesia time, presence of postoperative delirium, PACU and hospital length of stay, and all-cause mortality. The primary outcome of this study was the rate of all-cause mortality at the longest follow-up available for patients with or without ePND. The secondary outcomes were postoperative delirium and PACU and hospital length of stay. Cohen’s kappa coefficient was calculated to assess agreement between the two researchers regarding literature selection and data extraction.
Internal validity and risk of bias assessment
The internal validity and risk of bias of the studies included in the meta-analysis were assessed by four reviewers (LB, MY, KK, and NE) according to the latest version of the new Cochrane tool ROBINS-E (Risk Of Bias In Non-randomized Studies - of Exposures) [14]. Risk of bias was assessed by four researchers (LB, MY, KK, and NE) using the Delphi method in two rounds, and disagreements were resolved by consensus. Differences in estimates were also resolved by consensus. Publication bias and small-study effects were assessed using Egger’s test (MedCalc Statistical Software, version 19.5.6, Belgium) [15] and funnel plot analyses. A systematic Grading of Recommendation, Assessment, Development and Evaluation (GRADE) approach was used to rate the certainty of the evidence. The baseline evidence level was high in the studies on prognostic factors [16].
Data analysis and synthesis
We used STATA 17 (StataCorp LLC, USA) and Cochrane Review Manager (RevMan version 5.3, Denmark) to perform the meta-analysis. Inter-study heterogeneity was assessed using the I2 and Cochrane Q tests. If the value was ≥ 50% and/or the P value was < 0.05, the effect estimate was considered significant for heterogeneity and a random-effects model was used. If necessary, data presented as medians were converted to the mean ± standard deviation (SD) [17]. The effect size for continuous data is expressed as the standardized mean difference (SMD; Hedges’ g) with a 95% CI. We used the Cochrane handbook recommendations to re-express SMDs using the rules of thumb for effect sizes (< 0.40 = small effect, 0.40–0.70 = moderate effect, > 0.70 = large effect) [18]. Binary research results were used to calculate the odds ratio (OR) with 95% CI using the inverse variance method (Mantel-Haenszel method). To understand the meaning of the OR in terms of changes in the number of events, we converted the OR into a risk ratio (RR) [19]. Statistical significance was set at P < 0.05 for hypothesis testing. Meta-regression analysis using the restricted maximum likelihood random-effects model was performed to assess whether the association between exposure and outcomes varied by scale type, age, sex, ASA status, postoperative delirium prevalence, type of anesthetics, operation time, and anesthesia time [20]. Covariates were first tested using a univariate model and all covariates that were available for most of the studies were assessed using a multivariate model. Due to the nature of the study design, case-control studies were excluded from the calculations on overall occurrence of ePND and mortality but were included in the meta-analysis to calculate the OR [21].
Sensitivity analysis
Sensitivity analyses were performed by analyzing only the studies with a moderate risk of bias.
Results
Baseline characteristics of the included studies
During the initial search, 920 articles were identified, and the full texts of 481 were reviewed for eligibility. A total of 16,028 patients from 22 studies were ultimately included in this systematic review and meta-analysis (Fig. 1) [22–43]. The major exclusions are reported in Supplementary Table 1 (Cohen’s kappa for study selection, 0.88; for data extraction, 0.93). The overall occurrence rate of ePND was 13% (1,979 of the 15,008 total patients from 21 studies, excluding the case-control study [23]), and the overall reported mortality rate was 1.4% (33 of the 2,359 patients from two studies [28,38]).
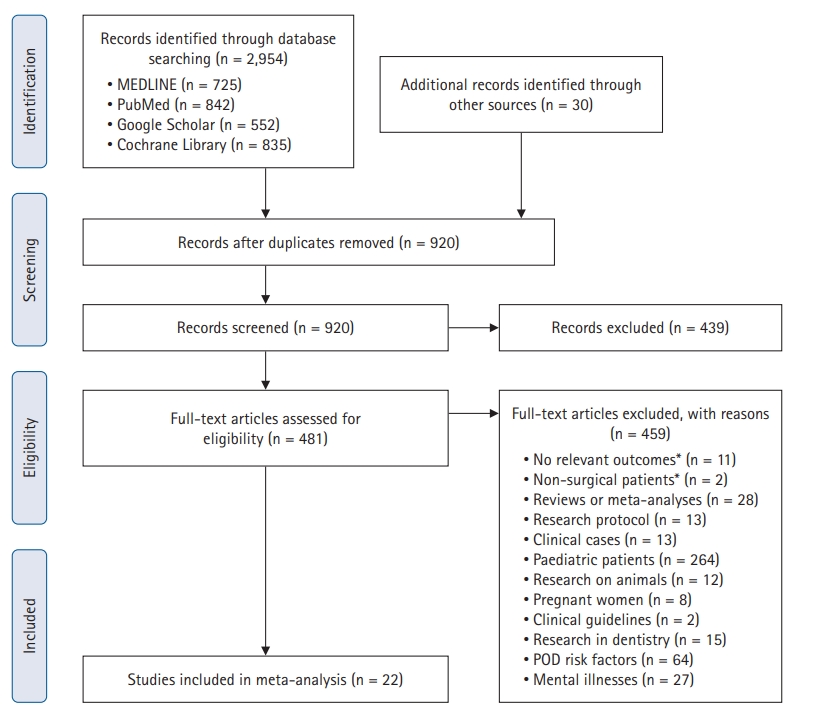
PRISMA flow diagram for study selection. POD: postoperative delirium. A detailed list of major exclusions is presented in the Supplement.
Among the 22 included studies, 20 were prospective observational [22,24,25,27–43] and eight used motor scales to assess awakening (9,277 patients, 58%) [22,23,26–28,34,40,41] (Table 1). The mean ages of the patients ranged from 39 to 79 years, and the proportion of patients with an ASA status ≥ 3 varied from 0% to 55%.
Quantitative data synthesis
All-cause mortality
Among the 22 included studies, three [23,28,38] (3,379 patients) reported all-cause mortality. The all-cause mortality was 16/677 (2.4%) in the ePND group and 32/2,702 (1.2%) in the normal emergence group (OR: 2.69, 95% CI [1.25, 5.76], RR: 2.6 [1.3, 5.5], P = 0.01; I2 = 0%) (Fig. 2, Table 2).
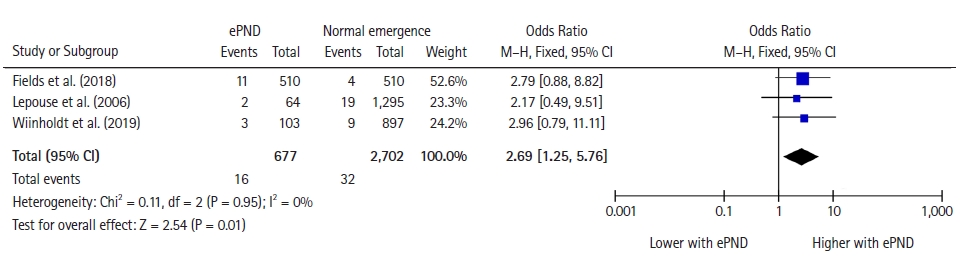
Forest plot for mortality representing the odds ratio for the effect of normal emergence versus early postoperative neurocognitive disorders (ePND) on all-cause mortality for the included studies. The plot displays the study, sample size, odds ratio, CI, and P value. The size of the squares indicates the weight of the studies (considering sample size and standard deviations); the diamond represents the pooled OR with CI.
Postoperative delirium
Postoperative delirium was reported in nine studies with 4,310 patients [23,24,26,30,32,34,37,41,43]. Patients with ePND, compared with those with normal emergence, had an increased incidence of postoperative delirium: 377/1,301 (29%) vs. 136/3,009 (4.5%) (OR: 15.72 [5.68, 43.53], RR: 9.5 [4.7, 14.9], P < 0.001; I2 = 93%) (Fig. 3, Table 2).
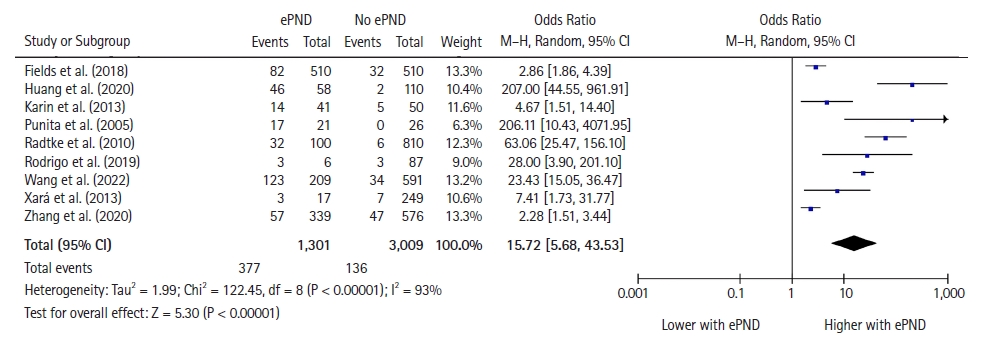
Forest plot for postoperative delirium representing the odds ratio for the effect of normal emergence versus early postoperative neurocognitive disorders (ePND) on postoperative delirium incidence for the included studies. The plot displays the study, sample size, odds ratio, CI, and P value. The size of the squares indicates the weight of the studies (considering sample size and standard deviations); the diamond represents the pooled OR with CI.
Publication bias and GRADE assessment
Egger’s test and the funnel plot analyses did not reveal small-study effects for the majority of the study outcomes (Table 2, Supplementary Figs. 5–8).
Owing to their observational nature, > 50% of studies had a high or very-high risk of bias for all studied outcomes (8/9 for postoperative delirium, 9/16 for length of PACU stay, 10/12 for length of hospital stay, and 2/3 for all-cause mortality) (Supplementary Figs. 1 and 2, Supplementary Table 2).
According to the GRADE approach, high quality evidence was only found for an increased risk of postoperative delirium in patients with ePND (Table 3).
Meta-regression
Multivariate meta-regression revealed no predictor of postoperative delirium or length of PACU stay (Supplementary Table 3); however, a meta-regression for all-cause mortality could not be performed due to the insufficient number of studies.
Discussion
Key findings
Our systematic review and meta-analysis of the available literature allowed us to document, for the first time, an increased mortality risk (RR = 2.6) in adult patients who experience ePND (very low quality of evidence). We also confirmed that ePND is associated with a 9-fold increase in postoperative delirium (high heterogeneity with I2 = 93%) and a prolonged PACU and hospital stay. However, the meta-regression analysis did not show an association between ePND and postoperative delirium.
Relationship with previous studies
Short- and long-term PNDs are associated with mortality [44]. ePND are a very early type of PND that can cause serious complications (e.g., aspiration pneumonia and hemorrhage) [28]. In addition, these complications often require physical restraints or pharmacological interventions that prolong hospitalization. Therefore, it is reasonable to assume that ePND are associated with increased mortality risk. The association between ePND and mortality has previously been suggested by Lepouse et al., who found a mortality of 4% vs. 1% in patients with and without ePND, respectively; however, the difference was not statistically significant due to the limited number of included patients [28].
Postoperative delirium is already a well-known predictor of mortality [45-47]. Our findings suggest that the presence of ePND is not a benign sign, but a harbinger of detrimental outcomes in terms of an increased risk of mortality and postoperative delirium, which warrants further prospective investigations.
Significance of study findings
Future studies should focus on prevention and treatment of ePND. Previous studies have suggested that non-pharmacological and pharmacological prevention and treatment may reduce the risk of emergence delirium in children [48]. However, evidence of this phenomenon in the adult population is lacking. Surgeons and anesthesiologists may consider these complications life-threatening and manage patients accordingly (e.g., prolonged or advanced postoperative monitoring, nonpharmacological preventive strategies for delirium, and early rehabilitation).
Strengths and limitations
Our study has some limitations. First, several studies included in the meta-analysis were classified as having a high risk of bias; however, the quality of evidence for the impact of ePND on the incidence of postoperative delirium was ‘high.’ Second, the included studies were heterogeneous from a clinical point of view (patient cohorts varied in age, sex, ASA status, type and duration of surgery, type of anesthetics, and incidence of postoperative delirium); however, this can also be considered a strength as this increases the external validity of our findings. Third, the findings of the meta-regression analyses should be interpreted with caution because of the small number of studies included in our meta-analysis [49]. Fourth, the inclusion of a case-control study [23] might have affected the strength of the association between ePND and clinical outcomes; however, baseline characteristics were matched between patients with and without emergence agitation [23], which could have enhanced the homogeneity between the two groups. Another strength of this study is the high number of included patients (16,028 patients over a 20-years period), 3,379 of which had vital status data.
Future studies and prospects
Consensus among physicians and researchers regarding the definition and classification of emergence delirium and postoperative delirium is needed. Currently, it is not clear whether they are different entities or refer to the same postoperative neurocognitive disorder occurring at different time points. Evered et al. [7] suggested combining them under the term ‘postoperative delirium’ regardless of the time of appearance, and the findings of our meta-analysis support this recommendation.
Because considerable confusion exists regarding the terms emergence agitation and emergence delirium, these terms should be defined more clearly. Most studies used emergence agitation and delirium interchangeably, while some researchers argued that emergence delirium does not exist, as it simply reflects continuation of the anesthetized state [50]. A consensus definition is necessary for the further investigation of ePND.
Our systematic review and meta-analysis showed that ePND increase the risk of all-cause mortality by 2.6 times and the risk of postoperative delirium by 9 times, suggesting that further study and clarification of the similarities and differences between ePND and postoperative delirium is needed.
Notes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
Availability of data: template data collection forms, data extracted from included studies, data used for all analyses on other data are available from the corresponding author on request.
Author Contributions
Valery V. Likhvantsev (Conceptualization; Writing – original draft; Writing – review & editing)
Giovanni Landoni (Conceptualization; Writing – original draft; Writing – review & editing)
Levan B. Berikashvili (Formal analysis; Writing – original draft)
Nadezhda V. Ermokhina (Formal analysis)
Mikhail Y. Yadgarov (Formal analysis; Writing – original draft)
Yuki Kotani (Formal analysis; Writing – original draft)
Kristina K. Kadantseva (Formal analysis)
Dmitry M. Makarevich (Formal analysis; Writing – original draft)
Andrey V. Grechko (Formal analysis; Writing – original draft)
Supplementary Materials
Search strategy for each database.
Excluded studies with a cause of exclusion.
Judgement of each domain in ROBINS-E tool.
Meta-regression.
Risk of bias evaluation of the included trials using ROBINS-E (The Risk Of Bias In Non-randomized Studies - of Exposure) tool.
Risk of bias assessment graph: proportion of studies with moderate, high or very high risk of bias for studied outcomes.
Forest plot for PACU length of stay. The plot displays the study, sample size, standardized mean difference (SMD), confidence interval (CI), and P value.
Forest plot for hospital length of stay. The plot displays the study, sample size, standardized mean difference (SMD), confidence interval (CI), and P value.
Funnel plot for mortality.
Funnel plot for postoperative delirium.
Funnel plot for PACU length of stay.
Funnel plot for hospital length of stay.
Bubble plot depicting the univariate meta regression of SMD (Hedges’g) with anesthesia time (for association between ePND and PACU LoS).