 |
 |
| Korean J Anesthesiol > Volume 77(1); 2024 > Article |
|
Abstract
Background
Data on the efficacy and incidence of adverse effects associated with dexmedetomidine (DEX) as a local anesthetic adjuvant for patient-controlled epidural analgesia (PCEA) are inconclusive. This meta-analysis assessed the efficacy and risks of DEX for PCEA using opioids as a reference.
Methods
Two researchers independently searched PubMed, Embase, Cochrane Library, and China Biology Medicine for randomized controlled trials comparing DEX and opioids as local anesthetic adjuvants in PCEA.
Results
In total, 636 patients from seven studies were included in this meta-analysis. Postoperative patients who received DEX had lower visual analog scale (VAS) scores than those who received opioids at 4ŌĆō8 h (mean difference [MD]: 0.61, 95% CI [0.45, 0.76], P < 0.001, I2 = 0%), 12 h (MD: 0.85, 95% CI [0.61, 1.09], P < 0.001, I2 = 0%), 24 h (MD: 0.59, 95% CI [0.06, 1.12], P = 0.030, I2 = 82%), and 48 h (MD: 0.54, 95% CI [0.05, 1.02], P = 0.030, I2 = 91%). Additionally, patients who received DEX had a lower incidence of itching (odds ratio [OR]: 2.86, 95% CI [1.18, 6.95], P = 0.020, I2 = 0%) and nausea and vomiting (OR: 6.83, 95% CI [3.63, 12.84], P < 0.001, I2 = 24%). In labor analgesia, no significant differences in neonatal (pH and PaO2 of cord blood, fetal heart rate) or maternal outcomes (duration of labor stage, mode of delivery) were found between the DEX and opioid groups.
Epidural analgesia is a widely practiced analgesic technique commonly used for postoperative analgesia, labor analgesia, and treatment of pain in the late stage of cancer, and has been demonstrated to improve postoperative outcomes and attenuate the physiological response to surgery [1ŌĆō6]. Patient-controlled epidural analgesia (PCEA) is a common form of epidural analgesia that provides better analgesia, improves patient satisfaction, and reduces clinician workload compared to continuous infusion and intermittent bolus techniques [7]. Opioids are often used as adjuvants in combination with local anesthetics for epidural analgesia as this provides significant synergistic effects [8]. The combined application prolongs the duration of analgesia, reduces the concentration and dosage of local anesthetics, and increases patient satisfaction [9]. However, the combined use of opioids and local anesthetics increases the incidence of side effects such as itching, urinary retention, nausea and vomiting, and respiratory depression [10ŌĆō12]. Therefore, the need for a more ideal adjuvant for local anesthesia, with better analgesic effects but fewer adverse effects, is growing. In recent years, several studies on the use of dexmedetomidine (DEX) as an adjuvant to local anesthetics for intravertebral anesthesia and analgesia have provided encouraging results.
DEX is a highly selective Alpha-2 receptor agonist with a variety of pharmacological effects including sedation, analgesia, and anti-sympathetic effects [13,14]. Several meta-analyses have demonstrated that the administration of DEX as an adjuvant in epidural anesthesia is well tolerated, acts synergistically, and provides an improved sedation and analgesic profile [15ŌĆō18]. Notably, some studies have also shown that administering DEX as a local anesthetic adjuvant in spinal anesthesia [19] and epidural anesthesia [20,21] not only prolongs the duration of anesthesia and improves postoperative analgesia, but also reduces the incidence of itching compared with opioids. However, in the abovementioned studies, the administration of DEX was typically as a single dose with a short duration of action. However, when administering continuous infusions of DEX through the PCEA over a prolonged period, careful consideration of the efficacy of the cumulative dose and possible adverse effects is essential.
Unfortunately, our current understanding of the adverse effects and efficacy of DEX administered to patients in PCEA is limited. In several randomized controlled trials (RCTs), DEX has been compared with opioids as adjuncts to local anesthetics for PCEA; however, the results have been inconsistent and even contradictory. Therefore, a meta-analysis is needed to evaluate the adverse effects and efficacy of DEX versus opioids as adjuvants in PCEA to provide insights into clinical analgesic practices.
The protocol for this meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42022307670 on March 10, 2022. This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards [22].
Two independent researchers (YFG and ZXC) searched PubMed, Embase, the Cochrane Library, and China Biology Medicine (CBM) for RCTs evaluating DEX as a local anesthetic adjuvant for PCEA from inception until January 10, 2022. The MeSH terms and free texts were used for study retrieval (Supplementary Marterial 1 for the retrieval strategy). The following terms were combined and used for the literature search: ŌĆ£dexmedetomidine,ŌĆØ ŌĆ£epidural space,ŌĆØ ŌĆ£analgesia, epidural,ŌĆØ ŌĆ£injections, epidural,ŌĆØ and ŌĆ£randomized controlled trial.ŌĆØ Furthermore, a manual search of the references of the included RCTs and systematic reviews in related fields was performed.
The eligibility criteria were based on the PICOS framework (participants, intervention, comparison, outcome, and study design) as follows: P: patients receiving PCEA (including postoperative and labor analgesia); I: use of DEX as an adjuvant to local anesthetics in PCEA; C: use of opioids as an adjuvant to local anesthetics in PCEA; O: visual analogue scale (VAS) scores and incidence of adverse effects as the primary outcomes and the number of PCEA bolus doses, total PCEA consumption, Ramsay sedation scale (RSS) scores, and neonatal and maternal outcomes (in labor analgesia) as secondary outcomes; and S: only RCTs were included. Our meta-analysis was divided into two groups for the qualitative synthesis: the DEX group and the opioid group (including but not limited to morphine, fentanyl, sufentanil, and hydromorphone).
Studies with any of the following characteristics were excluded: (1) a single-dose injection of DEX, (2) serious flaws in the study design, (3) incomplete and duplicate publications, (4) unretrievable or unconvertable data; and (5) not written in either English or Chinese.
Two evaluators (YFG and YH) independently screened the literature and extracted and cross-checked the data. In cases of disagreement, a third party (SJS) was consulted. During literature screening, the title and abstract were read first. After excluding irrelevant studies, the full texts of the remaining studies were read. Data extraction mainly included relevant information concerning the predefined primary outcomes (i.e., VAS scores and incidence of adverse effects) and secondary outcomes (i.e., RSS scores, number of PCEA bolus doses, PCEA consumption, and neonatal and maternal outcomes in labor analgesia). When necessary, the data were extracted from graphs or figures. To obtain further information or answers to queries concerning the data, we contacted the authors of the studies listed. When the units used for the outcome indicators differed across the included studies, we converted them to the same units; for example, we consistently converted hours to minutes.
Two evaluators (YFG and SJS) assessed the risk of bias in the included studies using the Cochrane Handbook version 5.0.2. For each included RCT, the adequacy of sequence generation, concealment of allocation, blind design, incomplete outcome data, selective reporting, and other risks of bias were assessed. Each item was classified as having ŌĆ£low deviation risk,ŌĆØ ŌĆ£high deviation risk,ŌĆØ or ŌĆ£unclear deviation risk.ŌĆØ Any discrepancies were resolved through discussion, and if necessary, a third researcher (DY) was consulted to resolve disagreements.
Two evaluators (ZXC and SJS) used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach to evaluate the quality of evidence. The evidence was rated as high, moderate, low, or very low according to the risk of bias, inconsistency, indirectness, imprecision, and publication bias. Disagreements were resolved through discussion or by referring to a third evaluator (DY).
The meta-analysis was performed using the RevMan 5.4 software (Nordic Cochrane Center). The binary outcomes are expressed using odds ratios (ORs) with 95% CIs and continuous outcomes are expressed as mean differences (MDs) or standardized mean differences (SMDs) with 95% CIs. The I2 test was used to examine the heterogeneity of the pooled results. When no statistical heterogeneity was present among the RCTs (I2 < 50%), a fixed-effects model was used to combine effect sizes. If statistical heterogeneity was present among the results (I2 Ōēź 50%), further sensitivity analyses and subgroup analyses were performed to evaluate the robustness of the synthesized results and to determine the source of heterogeneity. If heterogeneity could not be ruled out, a random effects model was used for the meta-analysis. The planned subgroup analysis assessed the use of PCEA for labor or postoperative analgesia. For the data expressed using the median and range, we obtained the corresponding means and standard deviations (SDs) using an online calculator (http://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html, last accessed on June 26, 2022) [23,24]. For data that were provided only as histograms, we obtained the specific means and SDs using a webplot digitizer (https://apps.automeris.io/wpd/index.zh_CN.html, last accessed on June 26, 2022).
As the observation indicators in the included studies were not completely consistent and the data results required to integrate the indicators were not included in some studies, the primary and secondary outcome results of our meta-analysis were integrated only for studies that contained data on the corresponding indicators. A brief description of some of the related indicators that were reported in the included studies but could not be integrated owing to insufficient data is provided in the Results section. A table combined with different custom symbols was used to visually display the effects of DEX versus opioids in PCEA based on the results of our meta-analysis.
A flowchart of the study selection is shown in Fig. 1. A total of 636 patients were included in the seven RCTs selected for this study [25ŌĆō31], including 320 and 316 patients treated with DEX and opioids, respectively. The basic characteristics and interventions are summarized in Table 1. Three studies [25,26,30] were conducted on patients receiving labor analgesia, and the remaining studies [27ŌĆō29,31] were conducted on patients receiving postoperative analgesia in surgical settings. The surgical procedures included cesarean sections [28], lumbar spine surgery [31], elective lung lobectomy [27], and colonic resections [29]. The risk of bias assessments are shown in Fig. 2. The quality of the meta-evidence on the efficacy and adverse effects of DEX in PCEA was generally low (Table 2).
Four trials, which included 335 patients, reported postoperative VAS scores at various time points (0ŌĆō4 h, 4ŌĆō8 h, 24 h, and 48 h), whereas three trials, which included 268 patients, reported VAS scores 12 h after surgery (Fig. 3). Significant heterogeneity was observed among the studies in the pooled analysis in the 24 h (P < 0.001, I2 = 82%) and 48 h (P < 0.001, I2 = 91%) groups. Besides the statistically insignificant results for the 0ŌĆō4 h group (MD: ŌłÆ0.13, 95% CI [ŌłÆ0.02, 0.27], P = 0.100), postoperative patients who received DEX reported lower VAS scores than those who received opioids in the 4ŌłÆ8 h (MD: 0.61, 95% CI [0.45, 0.76], P < 0.001), 12 h (MD: 0.85, 95% CI [0.61, 1.09], P < 0.001), 24 h (MD: 0.59, 95% CI [0.06, 1.12], P = 0.030), and 48 h (MD: 0.54, 95% CI [0.05, 1.02], P = 0.030) groups. These results indicate that DEX can significantly prolong postoperative analgesia and reduce postoperative pain compared to opioids.
In this section, we compare the incidence of adverse effects in postoperative patients, including hypotension, bradycardia, itching, urinary retention, nausea and vomiting, and shivering for DEX vs. opioids. Only one study [31] reported the incidence of dry mouth (DEX vs. fentanyl: 5/30 vs. 0/30).
Hypotension: Eight trials, which included 636 patients, reported the incidence of hypotension after the administration of DEX or opioids. A total of 320 patients received DEX for postoperative analgesia and 316 received opioids. Heterogeneity among the studies was significant in the pooled analysis (P = 0.040, I2 = 58%). The OR was 1.21 (95% CI [0.31, 4.81], P = 0.780), and the incidence of hypotension was comparable between the DEX and opioid groups for postoperative analgesia (Fig. 4A).
Bradycardia: Eight trials, which included 636 patients, reported the incidence of bradycardia after the administration of DEX or opioids. A total of 320 patients received DEX for postoperative analgesia and 316 patients received opioids. Heterogeneity among the studies was significant in the pooled analysis (P = 0.030, I2 = 63%). The OR was 0.35 (95% CI [0.05, 2.25], P = 0.270), and the incidence of bradycardia was similar between the DEX and opioid groups for postoperative analgesia (Fig. 4B).
Itching: Seven trials, which included 576 patients, reported itching after the administration of DEX or opioids. A total of 290 patients received DEX for postoperative analgesia and 286 received opioids. Heterogeneity among the studies was not significant in the pooled analysis (P = 0.420, I2 = 0%). The OR was 2.86 (95% CI [1.18, 6.95], P = 0.020), and the incidence of itching was significantly lower with DEX than with opioids for postoperative analgesia (Fig. 4C).
Urinary retention: Three trials, which included 231 patients, reported the incidence of urinary retention after the administration of DEX or opioids. A total of 116 patients received DEX for postoperative analgesia and 115 received opioids. No significant heterogeneity was observed among the studies in the pooled analysis (P = 0.170, I2 = 43%). The OR was 2.02 (95% CI [0.87, 4.71], P = 0.100), and the incidence of urinary retention was similar between the DEX and opioid groups for postoperative analgesia (Fig. 4D).
Nausea and vomiting: Eight trials, which included 636 patients, reported the incidence of nausea and vomiting after the administration of DEX or opioids. A total of 320 patients received DEX for postoperative analgesia and 316 received opioids. No significant heterogeneity was observed among the studies in the pooled analysis (P = 0.240, I2 = 24%). The OR was 6.83 (95% CI [3.63, 12.84], P < 0.001), and the incidence of nausea and vomiting was significantly lower with DEX compared to opioids for postoperative analgesia (Fig. 4E).
Shivering: Five trials, which included 409 patients, reported shivering after the administration of DEX or opioids. A total of 206 patients received DEX for postoperative analgesia and 203 received opioids. Heterogeneity among the studies was not significant in the pooled analysis (P = 0.810, I2 = 0%). The OR was 1.57 (95% CI [0.63, 3.95], P = 0.340), and the incidence of shivering was comparable between the DEX and opioid groups for postoperative analgesia (Fig. 4F).
Two trials, which included 199 patients, reported the number of PCEA bolus doses administered after the administration of DEX or opioids. A total of 99 patients received opioids and 100 received DEX. Heterogeneity among the studies was significant in the pooled analysis (P = 0.060, I2 = 73%). The MD was 3.40 (95% CI [1.61, 5.19], P <0.001), and the number of PCEA bolus doses was dramatically lower with DEX compared with opioids (Fig. 5A).
Three trials, which included 269 patients, reported the consumption of analgesics in PCEA after the administration of DEX or opioids. A total of 133 patients received opioids and 136 received DEX. Heterogeneity among the studies was significant in the pooled analysis (P < 0.001, I2 = 93%). The MD was 14.85 (95% CI [3.99, 25.71], P = 0.007), and the consumption of analgesics in PCEA was significantly lower with DEX than with opioids (Fig. 5B).
Three trials, which included 268 postoperative patients, reported RSS scores at various time points after surgery (0ŌĆō2 h, 2ŌĆō6 h, 12 h, 24 h, and 48 h) (Fig. 6). Heterogeneity was observed among the studies in the pooled analysis of the 2ŌĆō6 h (P = 0.070, I2 = 62%), 24 h (P = 0.001, I2 = 85%), and 48 h (P = 0.001, I2 = 85%) groups. Statistically significant differences in the RSS scores were found between the postoperative patients who received opioids and those who received DEX in the 0ŌłÆ2 h (MD: ŌłÆ0.35, 95% CI [ŌłÆ0.58, ŌłÆ0.12], P = 0.003), 2ŌłÆ6 h (MD: ŌłÆ0.23, 95% CI [ŌłÆ0.46, ŌłÆ0.00], P = 0.050), 12 h (MD: ŌłÆ0.79, 95% CI [ŌłÆ0.91, ŌłÆ0.67], P < 0.001), and 24 h (MD: ŌłÆ0.60, 95% CI [ŌłÆ0.96,ŌłÆ0.24], P = 0.001) groups. In the 48 h group (MD: ŌłÆ0.32, 95% CI [ŌłÆ0.66, 0.01], P = 0.060), no significant difference in the RSS scores were found between the postoperative patients who received DEX compared to those who received opioids. Overall, our results showed that patients treated with DEX in the early postoperative period had significantly higher levels of sedation than those treated with opioids. However, no significant difference was found at 48 h postoperatively.
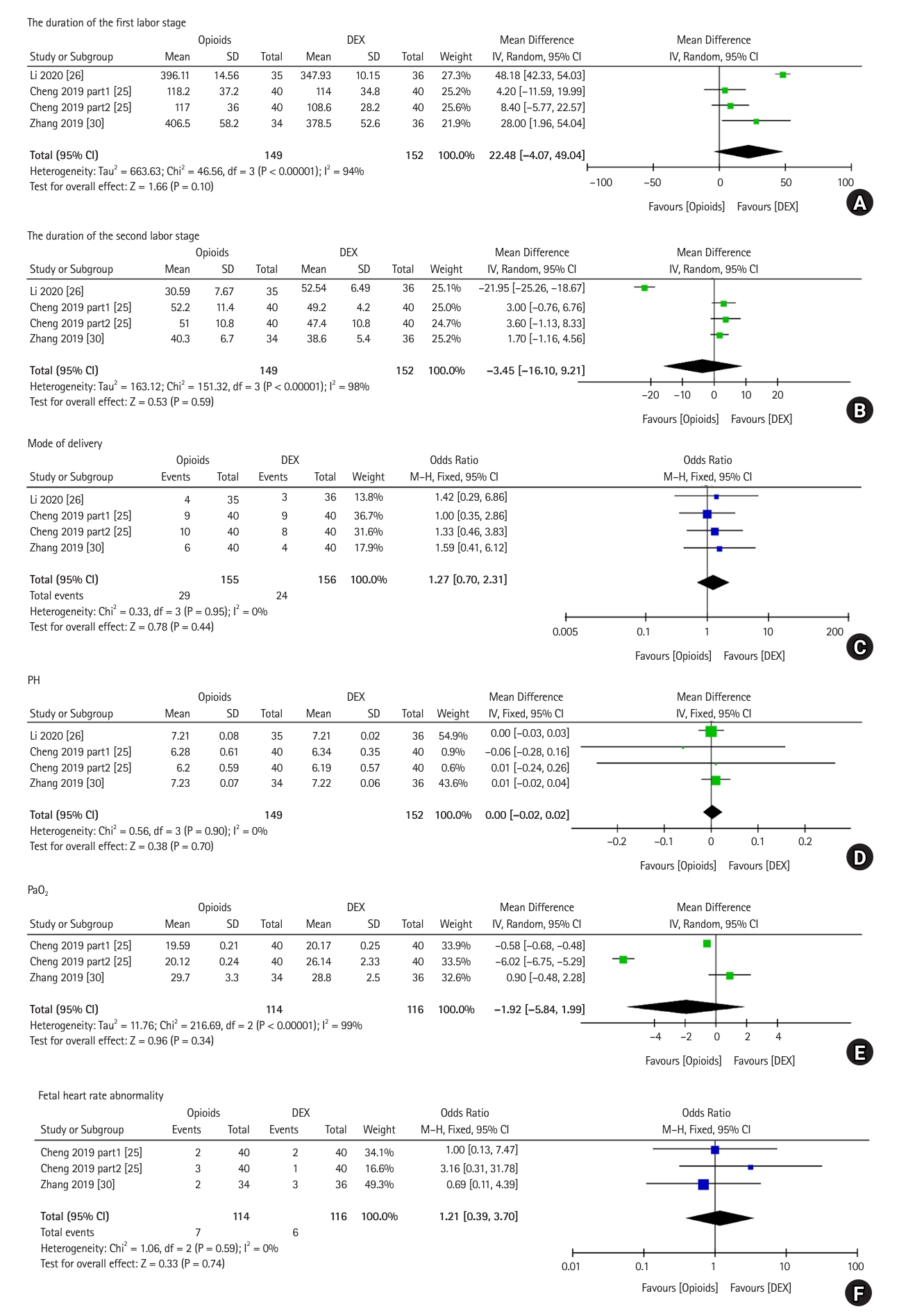
Four trials, which included 301 patients, compared the effects of DEX vs. opioids on the duration of each labor stage for labor analgesia. Significant heterogeneity was observed among the studies in the pooled analysis for both labor stages (first labor stage, P < 0.001, I2 = 94%; second labor stage, P < 0.001, I2 = 98%). The duration of the first (MD: 22.48, 95% CI [ŌłÆ4.07, 49.04], P = 0.100) and second (MD: ŌłÆ3.45, 95% Cl [ŌłÆ16.10, 9.21], P = 0.590) labor stages were similar between the groups, suggesting no significant difference in the effect of DEX and opioids on the duration of the labor stages for labor analgesia (Figs. 7A and B).
Four trials, which included 301 patients, reported the mode of delivery after the administration of DEX or opioids for labor analgesia. A total of 156 patients received DEX for labor analgesia and 155 received opioids. Heterogeneity among the studies was significant in the pooled analysis (P = 0.950, I2 = 0%). The OR was 1.27 (95% CI [0.70, 2.31], P = 0.440), and the percentage of vaginal deliveries was comparable between the DEX and opioid groups for labor analgesia (Fig. 7C).
Four trials, which included 301 patients, reported the pH of cord blood after the administration of DEX or opioids for labor analgesia. A total of 152 patients received DEX and 149 received opioids for labor analgesia. Heterogeneity among the studies was not significant in the pooled analysis (P = 0.90, I2 = 0%). The MD was 0.00 (95% CI [ŌłÆ0.02, 0.02], P = 0.70), and the pH of neonatal blood was similar between the DEX and opioid groups for labor analgesia (Fig. 7D).
Three trials, which included 230 patients, reported the PaO2 of cord blood. A total of 116 patients received DEX for labor analgesia and 114 received opioids. Significant heterogeneity was observed among the studies in the pooled analysis (P < 0.001, I2 = 99%). The MD was ŌłÆ1.92 (95% CI [ŌłÆ5.84, 1.99], P = 0.34), and the PaO2 of cord blood was similar between the DEX and opioid groups for labor analgesia (Fig. 7E).
Three trials, which included 230 patients, reported the incidence of fetal heart rate abnormalities after the administration of DEX or opioids for labor analgesia. A total of 116 patients received DEX for labor analgesia and 114 received opioids. No significant heterogeneity was observed among the studies in the pooled analysis (P = 0.59, I2 = 0%). The OR was 1.21 (95% CI [0.39, 3.70], P = 0.74) and the incidence of fetal heart rate abnormalities was similar between the DEX and opioid groups for labor analgesia (Fig. 7F).
Sensitivity and subgroup analyses were performed to minimize and identify the sources of heterogeneity. For some indicators, the heterogeneity was significantly reduced among the remaining studies after one study was removed (Table 3). For the VAS scores at 24 h postoperatively, RSS scores at various postoperative times (0-2 h, 2-6 h, 24 h, and 48 h), duration of the first labor stage, mode of delivery, and sensitivity analysis results using a fixed-effects model for pooling were consistent with previous results.
However, though the incidence of hypotension and bradycardia and the duration of the second labor stage were not significantly different between the opioid and DEX groups before the sensitivity analysis, after minimizing the heterogeneity, we concluded that a relatively higher incidence of bradycardia (OR: 2.27, 95% CI [1.05, 4.91], P = 0.04), lower incidence of hypotension (OR: 0.12, 95% CI [0.03, 0.54], P = 0.005), and relatively shorter second labor stage duration (MD: 2.44, 95% CI [0.39, 4.50], P = 0.02) were found in the DEX group compared with the opioid group. Furthermore, we performed a subgroup analysis of the incidence of hypotension and bradycardia based on whether PCEA was used for labor or postoperative analgesia. The results of this subgroup analysis were consistent with the previous unadjusted results. Therefore, we looked at these results dialectically. Unfortunately, even though we performed sensitivity and subgroup analyses, considerable heterogeneity remained for some of the results (VAS scores for postoperative patients at 48 h, PaO2 of cord blood, etc.); thus, future high-quality studies with larger sample sizes are needed to further confirm these results. The efficacy of DEX vs. opioids in the PCEA is summarized in Table 4.
This meta-analysis included seven studies, one of which (Cheng et al. [25]) contained two separate trials. The results of the present meta-analysis showed that DEX was superior to opioids in terms of pain relief and the incidence of nausea and vomiting and itching.
The VAS score is a critical parameter for evaluating the efficacy of potential analgesic therapies. This meta-analysis found that the addition of DEX resulted in lower VAS scores than the addition of opioids at 4ŌĆō8 h, 12 h, and 48 h after surgery. One curious finding was that DEX was not significantly superior to opioids at reducing VAS scores in patients 0ŌĆō4 h after surgery, which may be explained by the fact that researchers often administer the first dose of local anesthetics in the epidural space to expand the analgesic plane when linking the PCEA pump, thus masking the synergistic analgesic effect of the adjuvant considerably in the early postoperative period. The analgesic effects of DEX may be explained by several potential mechanisms. DEX opens the K+ channel on the cell membrane and strengthens the inhibitory effect of local anesthetics on the Na+ channel [32]. However, DEX can also inhibit the secretion of norepinephrine by the spinal cord, activate cholinergic nerves, and synergize with local anesthetics to enhance the analgesic effect [33]. In addition, DEX can induce local vasoconstriction by stimulating the Alpha-2 receptors of blood vessels, thereby delaying the absorption of local anesthetics and prolonging their duration [34]. In the meta-analysis, epidural DEX significantly reduced postoperative pain compared to opioids in terms of the VAS score; however, data on labor analgesia were not included, mainly because the time points for the VAS score evaluations in the included studies on labor analgesia varied and thus could not be integrated. In the study conducted by Zhang et al. [30], the progression of the cervical opening diameter was used as the observation point for the VAS score. Both Li et al. [26] and Cheng et al. [25] recorded VAS scores after anesthesia induction, but the observation time points were inconsistent. However, all included studies showed a tendency toward better pain control with DEX than with opioids for epidural labor analgesia. The results of the study conducted by Li et al. [26] demonstrated that the VAS scores were significantly lower in the DEX group than in the opioid group 10 min after epidural placement. Cheng et al. [25] showed the same results 15 min after anesthesia induction, and Zhang et al. [30] similarly showed lower VAS scores in the DEX group after cervical dilation > 3 cm. In a recently published meta-analysis [17] on epidural labor analgesia, the administration of DEX as a single shot or continuous infusion showed pain relief that was comparable with that of opioids. More high-quality research is needed in the future to draw clear conclusions on epidural labor analgesia.
The greatest concern with DEX administration in the epidural space is the potential for adverse effects, especially when used in PCEA because of its longer duration of action. DEX is associated with side effects such as hypotension, bradycardia, itching, dry mouth, shivering, nausea, and vomiting [35,36]. However, our analysis did not show a significantly higher risk of these side effects in the DEX group compared with the opioid group, except for dry mouth, though this is likely because an insufficient amount of studies reported the incidence of dry mouth. Furthermore, DEX significantly reduced the incidence of itching and nausea and vomiting compared with opioids. However, in our leave-one-out analysis, we found that the incidence of bradycardia was higher in the DEX group, whereas in the subgroup analysis of labor and postoperative analgesia, the incidence of bradycardia did not differ between the two groups. In another meta-analysis comparing DEX and placebo, Zhang et al. [15] found that the heart rate was lower in the DEX group; however, the incidence of bradycardia was not statistically significant. In conclusion, our results suggest that DEX is well-tolerated as an epidural adjuvant in PCEA and is superior to opioids in terms of the incidence of pruritus, nausea, and vomiting.
Our results indicate that DEX, when used as an adjuvant in PCEA, clearly increases the RSS scores in the early postoperative period. In studies comparing DEX with clonidine [37ŌĆō39], DEX significantly improved the degree of sedation. Relevant studies have demonstrated that DEX can diffuse into the cerebrospinal fluid through the dura mater and combine with Alpha-2 adrenergic receptors in the blue nucleus of the brainstem to produce a sedative effect [40]. Moderately increased sedation can help reduce the patientŌĆÖs stress response, reduce the bodyŌĆÖs oxygen consumption, maintain hemodynamic stability, and reduce dependence on analgesics that depress respiration. However, excessive sedation may cause side effects such as hypotension, bradycardia, and respiratory depression. As the use of DEX may be complicated by its higher sedative effect, careful attention by physicians is imperative during its use. Moreover, the addition of DEX reduces the consumption of anesthetics and PCEA bolus doses, reflecting the superiority of its analgesic effect laterally. Furthermore, compared with opioids, epidural analgesia with DEX has no significant impact on the duration of the stages, mode of delivery, umbilical artery pH or PaO2, or fetal heart rate. These findings are consistent with those of another previous study [17] that demonstrated that using DEX in epidural labor analgesia is safe for the fetus.
Previous meta-analyses have demonstrated that a single-shot injection of DEX into the subarachnoid or epidural space prolongs the duration of analgesia and decreases the requirement for rescue analgesia compared to placebo [15,16,18], opioids [41], or clonidine [42] in different surgical procedures. The most important difference between that study and the current meta-analysis is that the purpose of our study was to assess the safety and efficacy of continuous DEX infusions for epidural analgesia and thus, only studies involving continuous DEX infusions for epidural analgesia were included. Previous meta-analyses [17,41] have demonstrated that a single dose of DEX with epidural anesthesia significantly reduces the incidence of nausea and vomiting compared to opioids, which is consistent with our results. Qian et al. [41] found that, compared with epidural opioid administration, epidural DEX administration significantly reduces the incidence of shivering, though our results showed no significant difference. These authors also found that the incidence of dry mouth in the DEX group was significantly higher than that in the fentanyl group; however, the data from our included studies were insufficient to draw a definitive conclusion. In addition, the incidence of itching was significantly lower in the DEX group than in the opioid group in our study.
This meta-analysis has several limitations. First, data from the included studies were relatively insufficient. The VAS and RSS scores only included the postoperative analgesia population and may not be applicable for labor analgesia. Previous studies have reported that DEX may be associated with adverse effects, such as dry mouth and unstable hemodynamic changes. These results could not be synthesized or analyzed due to insufficient data in the studies included in this meta-analysis. Future studies should focus on these aspects. Second, this meta-analysis included studies using three different types of opioids (morphine, sufentanil, and fentanyl), which may have affected the results on pain relief and adverse events. Third, relatively high heterogeneity was found for some of our results, which may have resulted in bias; therefore, further research is required. The relatively high heterogeneity in this study may be explained by the different puncture segments among studies on epidural analgesia, different doses of DEX, and different PCEA parameter settings. Finally, DEX is a relatively new drug, particularly for epidural analgesia, and its long-term effects on the nervous system and maternal lactation are unknown. Therefore, suitable large-scale controlled trials to confirm these results are still necessary in the future.
In conclusion, the results of this meta-analysis showed that DEX is superior to opioids as a local anesthetic adjuvant in PCEA as DEX is associated with better pain relief and a lower incidence of nausea and vomiting and itching. However, some of the results of our meta-analysis should be interpreted with caution given the heterogeneity of the studies and insufficient data.
NOTES
Funding
This study was supported by the Wu Jieping Medical Foundation (No. 320.6750.2020-21-12). The sponsor was not involved in the study design; collection, management, analysis, and interpretation of data; writing of the report; or decision to submit the report for publication.
Fig.┬Ā2.
Risk of bias summary (A) and graph (B) of the included studies. None of the studies had a high risk of bias.
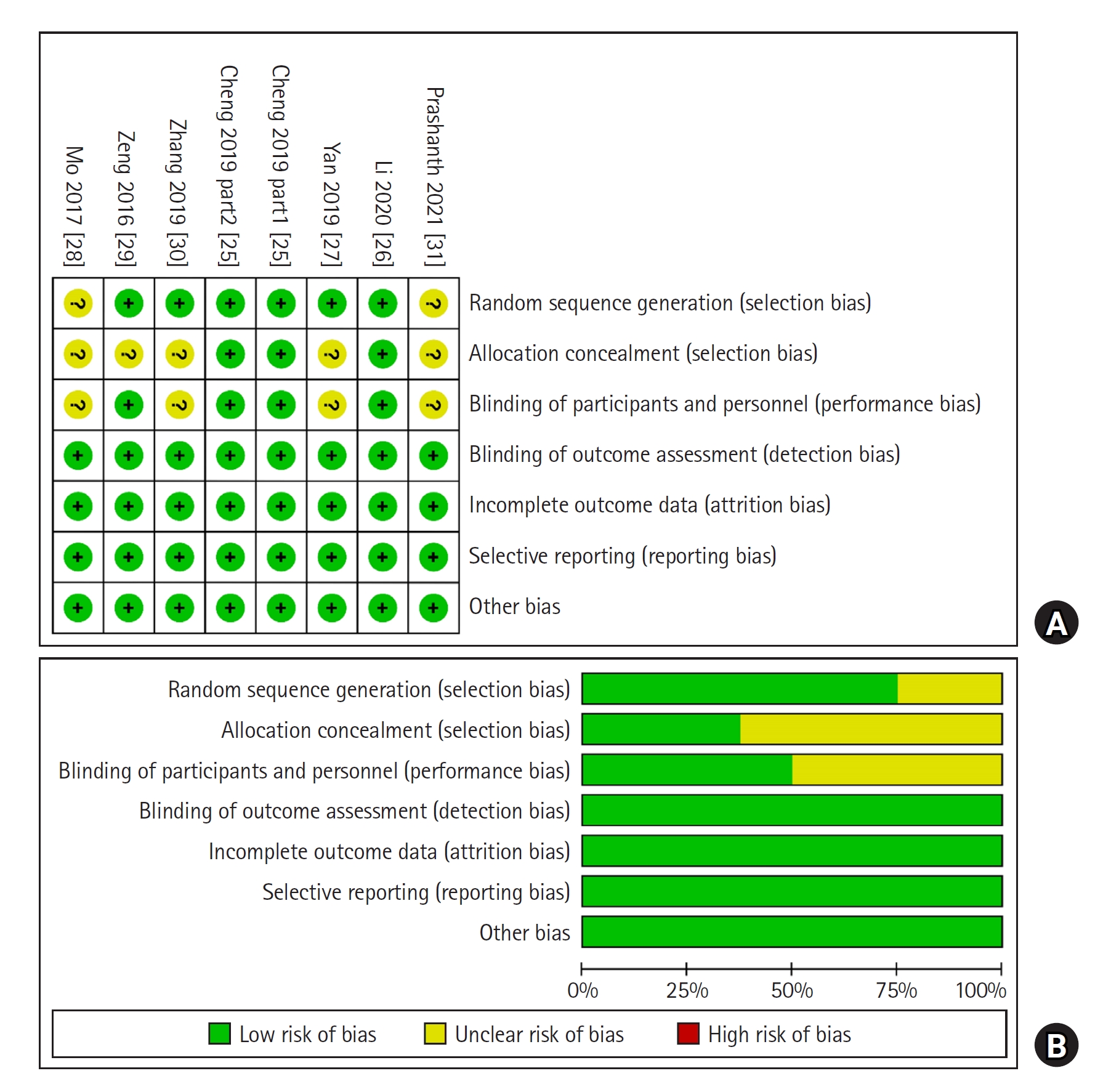
Fig.┬Ā3.
Forest plot of VAS scores for postoperative patients at various time points. VAS scores at (A) 0-4 h, (B) 4-8 h, (C) 12 h, (D) 24 h, and (E) 48 h are shown. DEX: dexmedetomidine, SD: standard deviation, IV: inverse variance, VAS: visual analog scale.
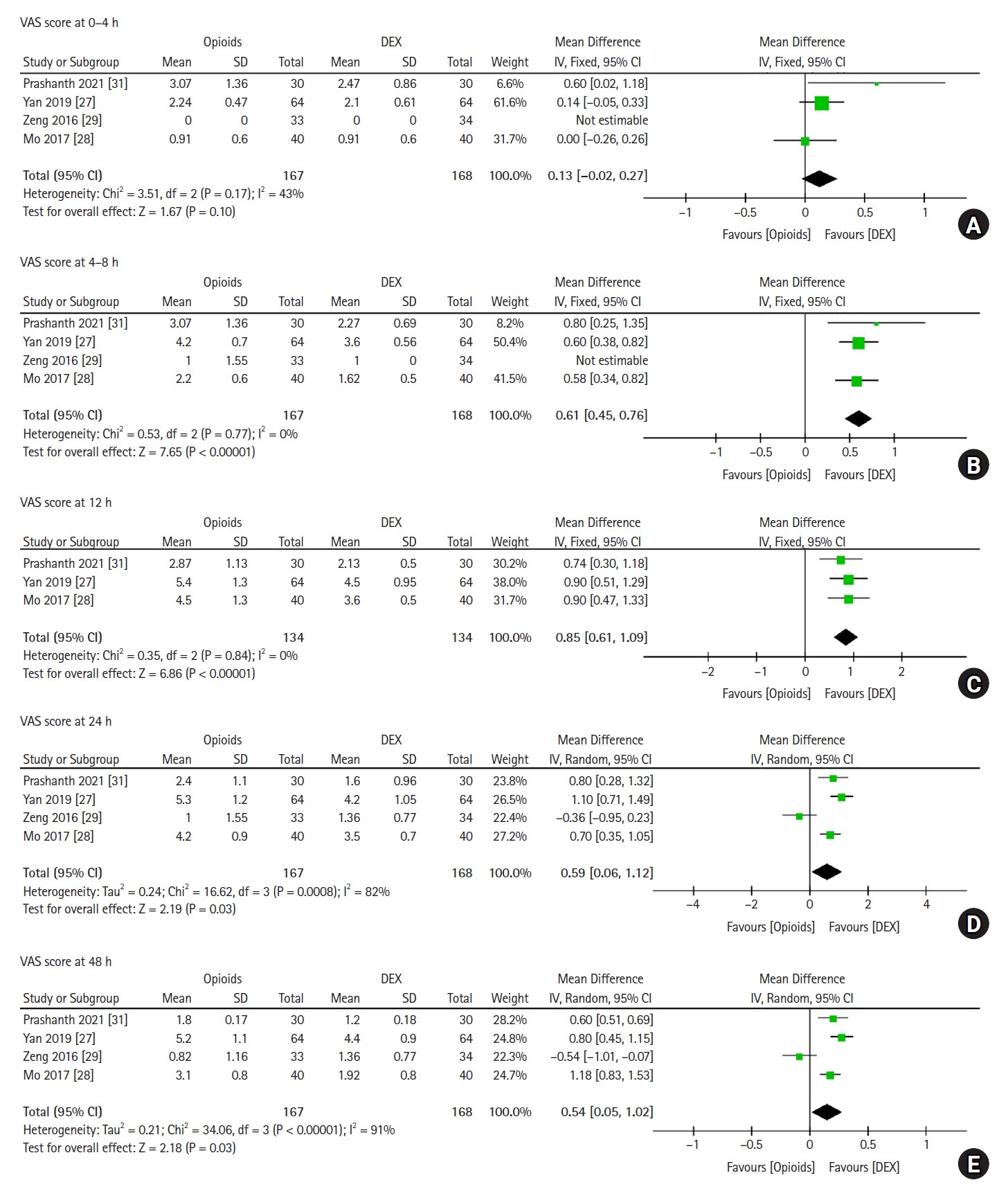
Fig.┬Ā4.
Forest plot comparing adverse effects between the DEX and opioid groups. (A) Hypotension, (B) bradycardia, (C) itching, (D) urinary retention, (E) nausea and vomiting, and (F) shivering. DEX: dexmedetomidine, M-H: Mantel-Haenszel.
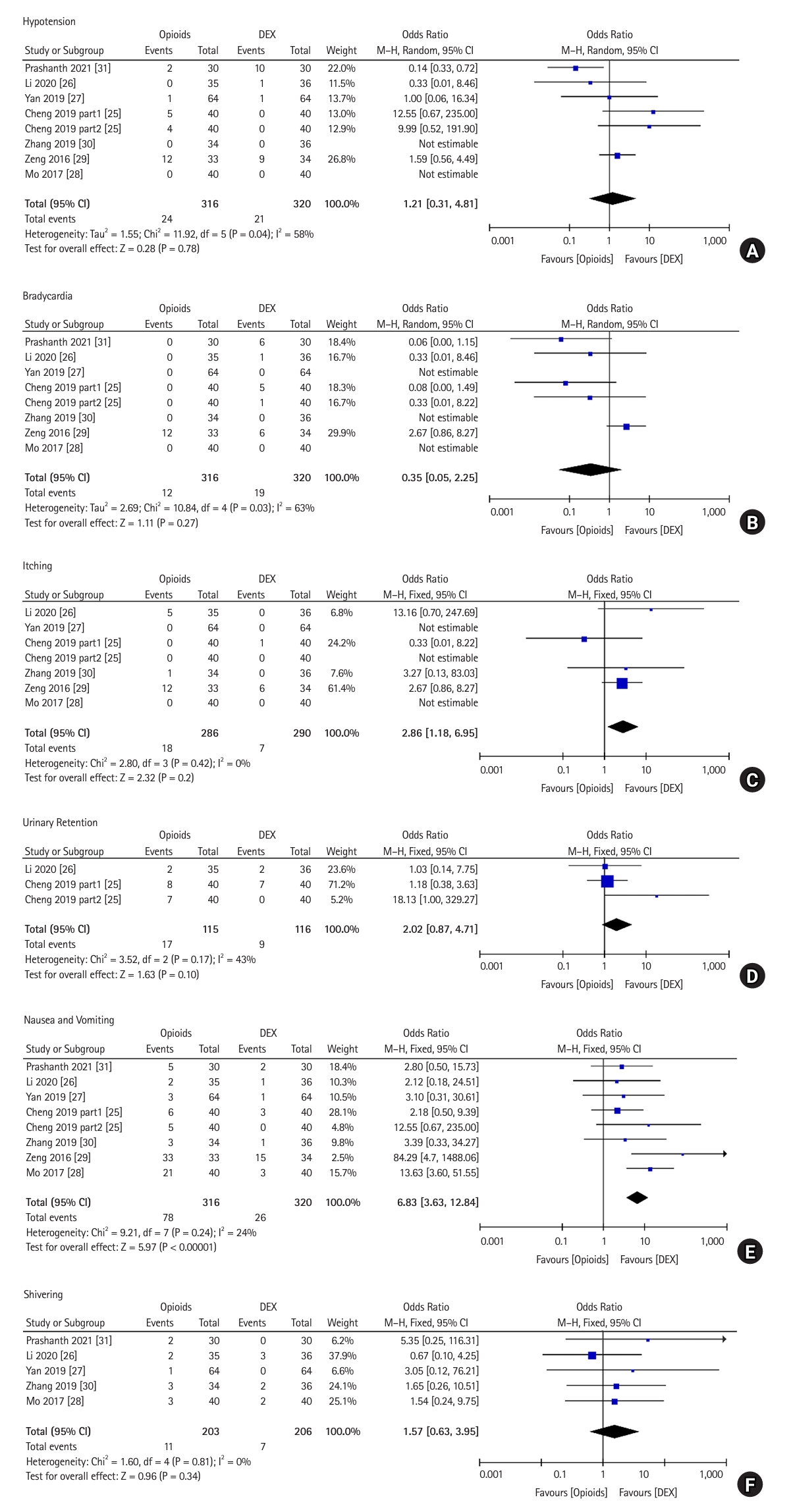
Fig.┬Ā5.
(A) Forest plot comparing the number of PCEA bolus doses and consumption of analgesics in PCEA between the DEX and opioid groups, (B) forest plot comparing the consumption of analgesics in PCEA between the DEX and opioid groups. DEX: dexmedetomidine, PCEA: patient-controlled epidural analgesia, IV: inverse variance.
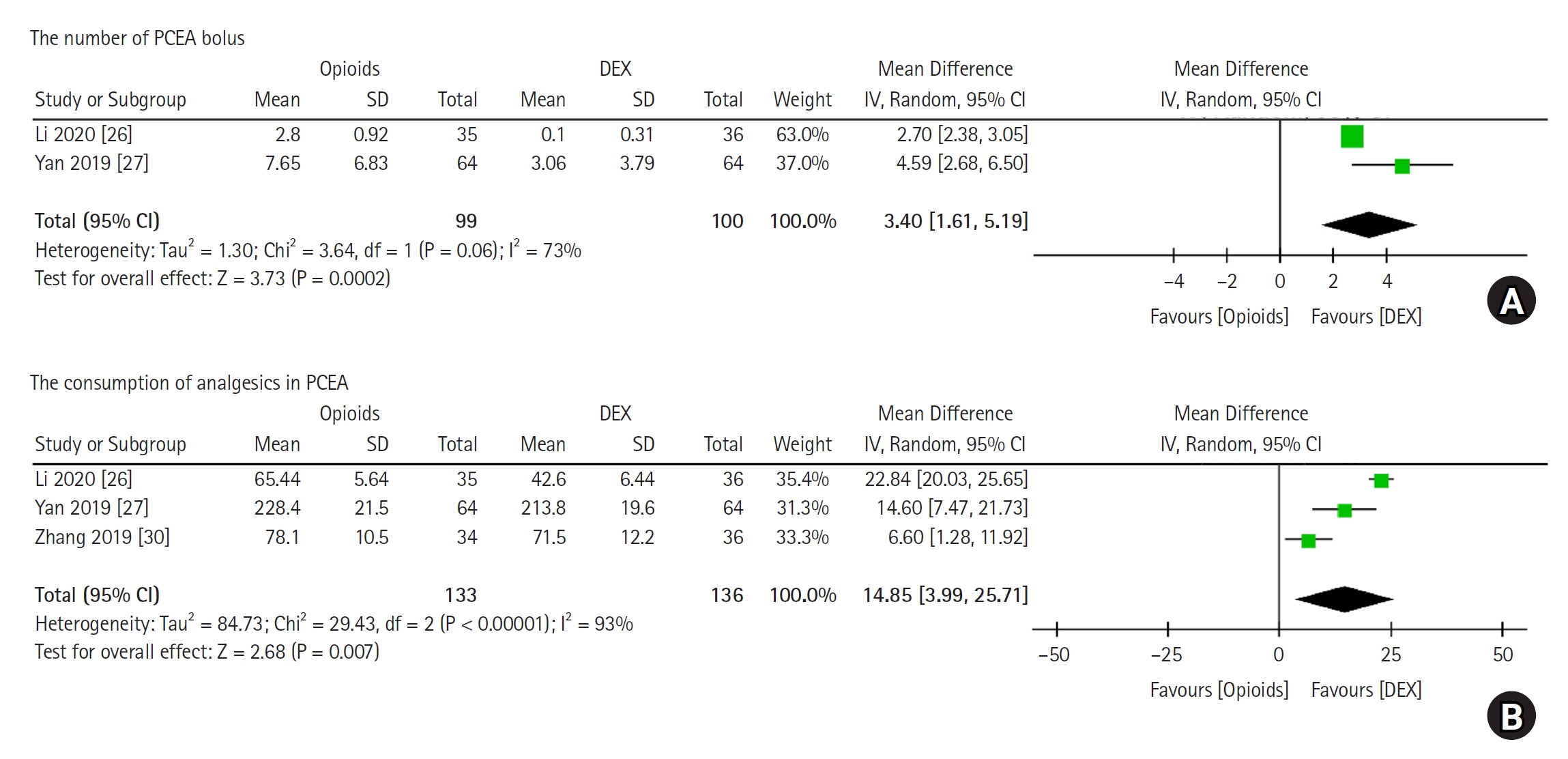
Fig.┬Ā6.
Forest plot of RSS scores for postoperative patients at various time points. RSS scores at (A) 0ŌĆō2 h, (B) 2ŌĆō6 h, (C) 12 h, (D) 24 h, and (E) 48 h are shown. RSS: Ramsay sedation scale, DEX: dexmedetomidine, SD: standard deviation, IV: inverse variance.
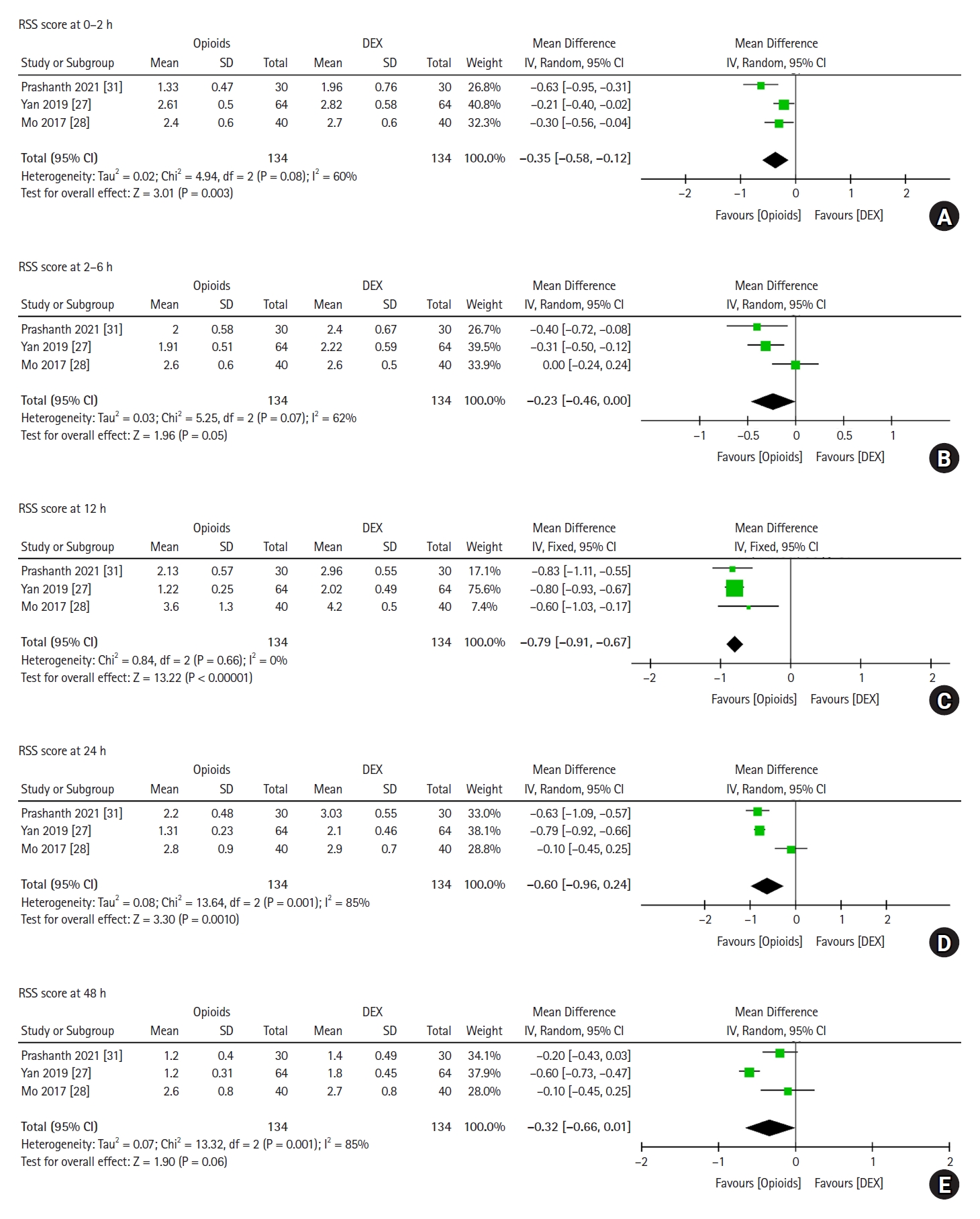
Fig.┬Ā7.
Forest plot of PCEA in labor analgesia. (A) Duration of the first labor stage, (B) duration of the second labor stage, (C) mode of delivery, (D) data on pH of cord blood, (E) data on PaO2 of cord blood, and (F) fetal heart rate abnormality. DEX: dexmedetomidine, IV: inverse variance, M-H: Mantel-Haenszel.
Table┬Ā1.
Basic Features of Included Studies
| Study (year) | Patients | Cases | Epidural catheter | Intervention | Dose | |
|---|---|---|---|---|---|---|
| Dexmedetomidine | Opioids | |||||
| Mo 2017 [28] | Cesarean section | 40/40 | - | DEX 1 ┬Ąg/kg | Morphine 5 mg | Loading dose: 5 ml |
| + 0.15% ropivacaine | + 0.15% ropivacaine | Background dose: 2 ml/h; bolus dose: 0.5 ml | ||||
| Zeng 2016 [29] | Colonic resection | 34/33 | T10-11 | DEX 80 ┬Ąg | Morphine 4.5 mg | Loading dose: 3 ml |
| + 0.125% levobupivacaine | + 0.125% levobupivacaine | Background dose: 3 ml/h | ||||
| Yan 2019 [27] | Elective lung lobectomy | 64/64 | T5-6 | DEX 0.5 ┬Ąg/ml | Sufentanil 0.5 ┬Ąg/ml | Loading dose: 4 ml |
| + 0.1% ropivacaine | + 0.1% ropivacaine | Background dose: 4 ml/h; bolus dose: 4 ml | ||||
| Prashanth 2021 [31] | Spine surgeries | 30/30 | L2-3/L3-4 | DEX 1 ┬Ąg/kg | Fentanyl 1 ┬Ąg/kg | Loading dose: 12 ml |
| + 0.2% ropivacaine | + 0.2% ropivacaine | Background dose: 5 ml/h | ||||
| Cheng 2019 part1 [25] | Full-term pregnancy | 40/40 | L3-4 | DEX 0.5 ┬Ąg/ml | Sufentanil 0.5 ┬Ąg/ml | Loading dose: 10 ml |
| + 0.125% ropivacaine | + 0.125% ropivacaine | Background dose: 8 ml/h; bolus dose: 8 ml | ||||
| Cheng 2019 part2 [25] | Full-term pregnancy | 40/40 | L3-4 | DEX 0.5 ┬Ąg/ml | Sufentanil 0.5 ┬Ąg/ml | Loading dose: 10 ml |
| + 0.08% ropivacaine | + 0.08% ropivacaine | Background dose: 8 ml/h; bolus dose: 8 ml | ||||
| Zhang 2019 [30] | Full-term pregnancy | 36/34 | L2-3 | DEX 0.5 ┬Ąg/ml | Sufentanil 0.5 ┬Ąg/ml | Loading dose: 10ml |
| + 0.1% ropivacaine | + 0.1% ropivacaine | Background dose: 6 ml/h; bolus dose: 6 ml | ||||
| Li 2020 [26] | Full-term pregnancy | 36/35 | L2-3 | DEX 0.5 ┬Ąg/ml | Ropivacaine 0.1% | Loading dose: 10ml |
| + 0.1% ropivacaine | + 0.5 ┬Ąg/ml sufentanil | Background dose: 7 ml/h; bolus dose: 7 ml | ||||
Table┬Ā2.
GRADE Evidence Result Summary
Table┬Ā3.
Sensitivity Analysis Results
Table┬Ā4.
Summary of the Efficacy of DEX vs. Opioids in PCEA
References
1. Moraca RJ, Sheldon DG, Thirlby RC. The role of epidural anesthesia and analgesia in surgical practice. Ann Surg 2003; 238: 663-73.



2. Esteve N, Ferrer A, Sansaloni C, Mariscal M, Torres M, Mora C. Epidural anesthesia and analgesia in liver resection: safety and effectiveness. Rev Esp Anestesiol Reanim 2017; 64: 86-94.


3. Capdevila X, Moulard S, Plasse C, Peshaud JL, Molinari N, Dadure C, et al. Effectiveness of epidural analgesia, continuous surgical site analgesia, and patient-controlled analgesic morphine for postoperative pain management and hyperalgesia, rehabilitation, and health-related quality of life after open nephrectomy: a prospective, randomized, controlled study. Anesth Analg 2017; 124: 336-45.


4. P├Čpping DM, Elia N, Van Aken HK, Marret E, Schug SA, Kranke P, et al. Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta-analysis of randomized controlled trials. Ann Surg 2014; 259: 1056-67.


5. Mohamad MF, Mohammad MA, Hetta DF, Ahmed EH, Obiedallah AA, Elzohry AA. Thoracic epidural analgesia reduces myocardial injury in ischemic patients undergoing major abdominal cancer surgery. J Pain Res 2017; 10: 887-95.



6. Ahn JH, Ahn HJ. Effect of thoracic epidural analgesia on recovery of bowel function after major upper abdominal surgery. J Clin Anesth 2016; 34: 247-52.


7. DŌĆÖAngelo R. New techniques for labor analgesia: PCEA and CSE. Clin Obstet Gynecol 2003; 46: 623-32.


8. Xiang B, Yang J, Lei X, Yu J. Adjuvant sufentanil decreased the ec50 of epidural ropivacaine for labor analgesia in healthy term pregnancy. Drug Des Devel Ther 2021; 15: 2143-9.




9. Guay J, Suresh S, Kopp S, Johnson RL. Postoperative epidural analgesia versus systemic analgesia for thoraco-lumbar spine surgery in children. Cochrane Database Syst Rev 2019; 1: CD012819.



10. Lorenzini C, Moreira LB, Ferreira MB. Efficacy of ropivacaine compared with ropivacaine plus sufentanil for postoperative analgesia after major knee surgery. Anaesthesia 2002; 57: 424-8.



11. Salicath JH, Yeoh EC, Bennett MH. Epidural analgesia versus patient-controlled intravenous analgesia for pain following intra-abdominal surgery in adults. Cochrane Database Syst Rev 2018; 8: CD010434.



12. Guay J, Kopp S. Epidural pain relief versus systemic opioid-based pain relief for abdominal aortic surgery. Cochrane Database Syst Rev 2016; 2016: CD005059.



13. Ng KT, Shubash CJ, Chong JS. The effect of dexmedetomidine on delirium and agitation in patients in intensive care: systematic review and meta-analysis with trial sequential analysis. Anaesthesia 2019; 74: 380-92.



14. Keating GM. Dexmedetomidine: a review of its use for sedation in the intensive care setting. Drugs 2015; 75: 1119-30.



15. Zhang X, Wang D, Shi M, Luo Y. Efficacy and safety of dexmedetomidine as an adjuvant in epidural analgesia and anesthesia: a systematic review and meta-analysis of randomized controlled trials. Clin Drug Investig 2017; 37: 343-54.


16. Zhao J, Liao C, Wu Q, Wang L, Deng F, Zhang W. Evaluation of ropivacaine combined with dexmedetomidine versus ropivacaine alone for epidural anesthesia: a meta-analysis. Medicine (Baltimore) 2021; 100: e25272.



17. Li N, Hu L, Li C, Pan X, Tang Y. Effect of epidural dexmedetomidine as an adjuvant to local anesthetics for labor analgesia: a meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med 2021; 2021: 4886970.




18. Wang XX, Dai J, Dai L, Guo HJ, Zhou AG, Pan DB. Caudal dexmedetomidine in pediatric caudal anesthesia: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2020; 99: e21397.



19. Sun S, Wang J, Bao N, Chen Y, Wang J. Comparison of dexmedetomidine and fentanyl as local anesthetic adjuvants in spinal anesthesia: a systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther 2017; 11: 3413-24.



20. Gousheh M, Akhondzadeh R, Rashidi M, Olapour A, Moftakhar F. Comparison of dexmedetomidine and morphine as adjuvants to bupivacaine for epidural anesthesia in leg fracture surgery: a randomized clinical trial. Anesth Pain Med 2019; 9: e91480.



21. Trifa M, Tumin D, Tobias JD. Dexmedetomidine as an adjunct for caudal anesthesia and analgesia in children. Minerva Anestesiol 2018; 84: 836-47.


22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097.



23. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018; 27: 1785-805.



24. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135.




25. Cheng Q, Bi X, Zhang W, Lu Y, Tian H. Dexmedetomidine versus sufentanil with high- or low-concentration ropivacaine for labor epidural analgesia: a randomized trial. J Obstet Gynaecol Res 2019; 45: 2193-201.



26. Li G, Xiao Y, Qi X, Wang H, Wang X, Sun J, et al. Combination of sufentanil, dexmedetomidine and ropivacaine to improve epidural labor analgesia effect: a randomized controlled trial. Exp Ther Med 2020; 20: 454-60.



27. Yan MJ, Wang T, Wu XM, Zhang W. Comparison of dexmedetomidine or sufentanil combined with ropivacaine for epidural analgesia after thoracotomy: a randomized controlled study. J Pain Res 2019; 12: 2673-8.



28. Mo Y, Qiu S. Effects of dexmedetomidine in reducing post-cesarean adverse reactions. Exp Ther Med 2017; 14: 2036-9.



29. Zeng XZ, Lu ZF, Lv XQ, Guo YP, Cui XG. Epidural co-administration of dexmedetomidine and levobupivacaine improves the gastrointestinal motility function after colonic resection in comparison to co-administration of morphine and levobupivacaine. PloS One 2016; 11: e0146215.



30. Zhang T, Yu Y, Zhang W, Zhu J. Comparison of dexmedetomidine and sufentanil as adjuvants to local anesthetic for epidural labor analgesia: a randomized controlled trial. Drug Des Devel Ther 2019; 13: 1171-5.



31. Prashanth G, Dash S, Mohapatra S, Moda N. Epidural ropivacaine and dexmedetomidine with that of epidural ropivacaine and fentanyl for postoperative analgesia in lumbar spine surgeries - a randomised double-blinded study. J Clin Diagn Res 2021; 15: UC01-4.

32. Brown EN, Pavone KJ, Naranjo M. Multimodal general anesthesia: theory and practice. Anesth Analg 2018; 127: 1246-58.



33. Weerink MA, Struys MM, Hannivoort LN, Barends CR, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet 2017; 56: 893-913.




34. Gallego-Ligorit L, Vives M, Vall├®s-Torres J, Sanju├Īn-Villarreal TA, Pajares A, Iglesias M. Use of dexmedetomidine in cardiothoracic and vascular anesthesia. J Cardiothorac Vasc Anesth 2018; 32: 1426-38.


35. Soni P. Comparative study for better adjuvant with ropivacaine in epidural anesthesia. Anesth Essays Res 2016; 10: 218-22.



36. Han C, Jiang X, Wu X, Ding Z. Application of dexmedetomidine combined with ropivacaine in the cesarean section under epidural anesthesia. Zhonghua Yi Xue Za Zhi 2014; 94: 3501-5.

37. Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: a comparative evaluation. Indian J Anaesth 2011; 55: 116-21.



38. Arunkumar S, Hemanth Kumar VR, Krishnaveni N, Ravishankar M, Jaya V, et al. Comparison of dexmedetomidine and clonidine as an adjuvant to ropivacaine for epidural anesthesia in lower abdominal and lower limb surgeries. Saudi J Anaesth 2015; 9: 404-8.



39. Channabasappa SM, Venkatarao GH, Girish S, Lahoti NK. Comparative evaluation of dexmedetomidine and clonidine with low dose ropivacaine in cervical epidural anesthesia for modified radical mastectomy: a prospective randomized, double-blind study. Anesth Essays Res 2016; 10: 77-81.



40. Sottas CE, Anderson BJ. Dexmedetomidine: the new all-in-one drug in paediatric anaesthesia? Curr Opin Anaesthesiol 2017; 30: 441-51.










 :means that DEX is better than opioids,
:means that DEX is better than opioids,  : means dexmedetomidine is worse than opioids,
: means dexmedetomidine is worse than opioids,  : means that DEX has the same effect as opioids. DEX: dexmedetomidine, PCEA: patient-controlled epidural analgesia.
: means that DEX has the same effect as opioids. DEX: dexmedetomidine, PCEA: patient-controlled epidural analgesia.



