 |
 |
| Korean J Anesthesiol > Volume 76(1); 2023 > Article |
|
Abstract
Background
Methods
Results
Conclusions
NOTES
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (No. 2018R1C1B6 004841).
This work was supported by clinical research grant from Pusan National University Hospital in 2022.
Conflicts of Interest
Eunsoo Kim has been an editor for the Korean Journal of Anesthesiology since 2016. However, he was not involved in any process of review for this article, including peer reviewer selection, evaluation, or decision-making. There were no other potential conflicts of interest relevant to this article.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Soeun Jeon (Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Writing – original draft; Writing – review & editing)
Jiseok Baik (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Supervision; Writing – original draft; Writing – review & editing)
Jisu Kim (Visualization; Writing – original draft)
Jiyoon Lee (Investigation; Visualization; Writing – original draft)
Wangseok Do (Conceptualization; Data curation; Visualization; Writing – original draft)
Eunsoo Kim (Supervision; Visualization; Writing – original draft)
Hyeon Jeong Lee (Investigation; Supervision; Writing – review & editing)
Haekyu Kim (Conceptualization; Methodology; Supervision)
Fig. 1.
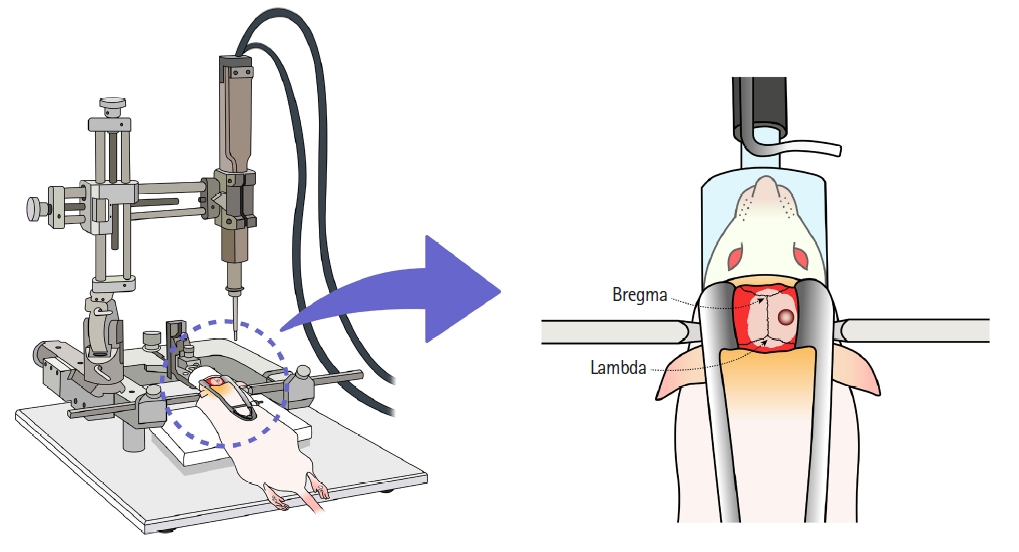
Fig. 2.

Fig. 3.

Fig. 4.
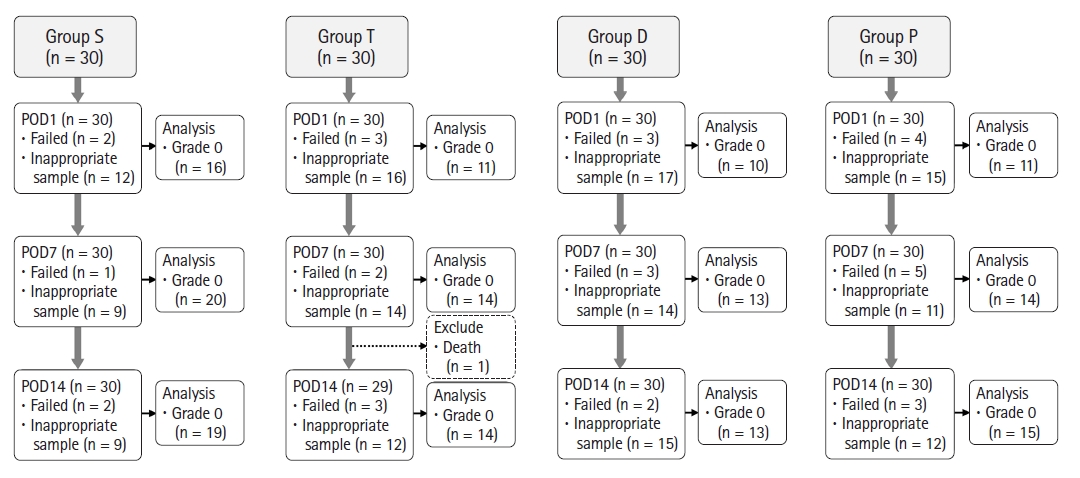
Fig. 5.
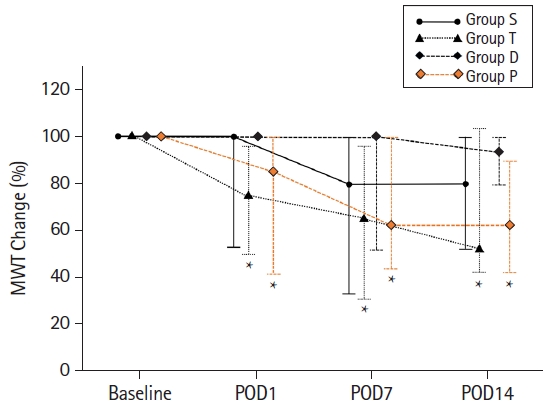
Fig. 6.

Table 1.
The normally distributed values are presented as mean ± SD (number), and non-parametric values are presented as median with first and third quartile [Q1, Q3]. The Tukey-Kramer test and Conover test were performed for post-hoc multiple comparisons as appropriate. Group S: sham group, group T: untreated traumatic brain injury (TBI), group D: dexmedetomidine treatment after TBI, group P: propofol treatment after TBI. POD: postoperative day, N/A: not applicable. *P < 0.05 compared to the group S, †P < 0.05 compared to group T.
Table 2.
Values are presented as median with first and third quartile [Q1, Q3] (number); and Conover test was performed for post-hoc multiple comparisons. Group S: sham group, group T: untreated traumatic brain injury (TBI), group D: dexmedetomidine treatment after TBI, group P: propofol treatment after TBI. 50% MWT: 50% mechanical withdrawal threshold, POD: postoperative day. *P < 0.05 compared to the group S, †P < 0.05 compared to group T.
Table 3.
Values are presented as median with first and third quartile [Q1, Q3] (number); and Conover tests were performed for post-hoc multiple comparisons. Group S: sham group, group T: untreated traumatic brain injury (TBI), group D: dexmedetomidine treatment after TBI, group P: propofol treatment after TBI. BDNF: brain-derived neurotrophic factor, CSF: cerebrospinal fluid, POD: postoperative day. *P < 0.05 compared to group T, †P < 0.05 compared to the group S.









