 |
 |
| Korean J Anesthesiol > Volume 74(1); 2021 > Article |
|
Transmission of severe acute respiratory syndrome coronavirus 2, leading to coronavirus disease (COVID-19) is thought to occur primarily through respiratory droplets, while airborne transmission may occur with the generation of aerosols. Upper gastrointestinal endoscopy is deemed a potentially infectious aerosol-generating procedure; consequently, healthcare workers present at the premises are at a high risk, particularly because of the physical proximity to the patients.
Various professional guidelines [1] recommend multi-pronged strategies for the use of endoscopes in the management of patients with COVID-19. This include deferring non-essential endoscopy, appropriate use of personal protective equipment (PPE), standard infection control practices, strict isolation precautions in negative-pressure rooms, and adequate disinfection protocols.
After obtaining written informed consent, we describe the management of a 30-year-old man who had a history of thalassemia intermedia and presented with a 1-day history of fever, sore throat, and myalgia. A positive nasopharyngeal swab test confirmed COVID-19.
On day 3, the patient developed productive cough. On day 7, he developed acute cholangitis. Imaging showed choledocholithiasis, likely a pigment stone secondary to chronic hemolysis. On day 18, in view of recurrent abdominal pain and worsening derangement of liver enzymes despite antibiotics, endoscopic retrograde cholangiopancreatography (ERCP) was performed instead of waiting for COVID-19 clearance, as was initially planned. As the patient was clinically stable with adequate oxygen saturation on room air, the procedure was performed under sedation.
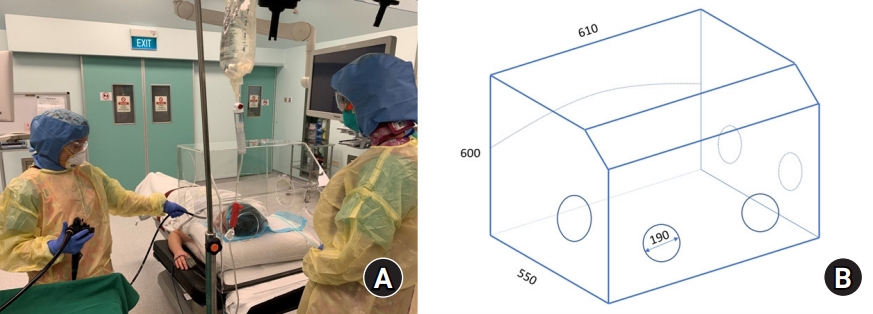
To reduce the risk of contamination, we used a barrier enclosure during the procedure. The barrier enclosure was a transparent acrylic trapezoidal box placed over the patient's head and upper torso during ERCP (Fig. 1A). It measured 60 ├Ś 55 ├Ś 61 cm (Fig. 1B).
The patient was initially placed in a supine position in the box, and lignocaine was sprayed to anesthetize the pharynx. The patient was then turned to a prone position in the box, and a nasal cannula was applied. Sedation was induced with titrated boluses of midazolam, fentanyl, and propofol.
Similar boxes, conceptualized for use with intubation, have garnered much interest in recent months. Kearsley [2] highlighted concerns regarding the use, while others speculated on the physics and virulence of droplets and aerosols in infection transmission [3], thus questioning the utility of such an enclosure.
The patient coughed when lignocaine was sprayed into the pharynx and gagged slightly at the start of the procedure. Droplet infectivity is multifactorial, depending on the droplet size, number, velocity, and viral load [3]. It is thought that ranges of droplet size and number are produced in a single coughing event. The smaller the size, the longer they remain suspended in the air. As large droplets dry, it is unknown if smaller aerosols are produced. Some aerosols are produced with normal breathing, while coughing produces aerosols profusely. The box may trap secretions and large respiratory droplets during the passage of the endoscope, suctioning, or patient coughing. Further, this may decrease the virus burden reaching the healthcare worker. In a simulation by Canelli et al. [4], the use of a barrier enclosure for intubation contained fluorescent dye within the inner surfaces of the enclosure, suggesting reduced macroscopic contamination of the immediate surroundings.
The effect of barrier devices on airborne particles, however, may be less benign. Simpson demonstrated that airborne transmission to the healthcare worker increased substantially when using an aerosol containment device [5]. Additionally, since the virus may remain viable in aerosols for 3 h [6], infectious aerosols can be released on box removal. Hence, the barrier should only be removed when the risk for aerosolization is deemed to be low and performed in a controlled manner to minimize the dispersal of viral particles.
The opening in the box held the endoscope in place after optimal positioning; however, with fixed openings, access to the patient for suctioning and repositioning was restricted. When the patient started moving excessively, an assistant reached the patient via the openings opposite the endoscopist while sedation was titrated. The openings allowed the anesthetist and assistant to intervene, but for patients with a high risk for deterioration, requirement of emergent intubation, or morbid obesity, the box may impede a quick access.
The procedure was completed in 1 h with the pigment stone removed and biliary stent inserted. The patient remained stable throughout. He was asked to turn to the supine position in the box, and a surgical mask was applied. The processes of box removal and decontamination were performed with much care to avoid contaminating the surroundings with droplets remaining on the inner surface of the box or the patient's blanket or bed.
The half-life of the virus on plastic is 6.5 h [6]; therefore, careful removal of the box and meticulous decontamination are imperative. It required considerable effort with numerous wipes used to ensure that every crevice was clean. A liquid or vapor disinfectant could serve as a more convenient decontamination method1). The postoperative course was uneventful, and the patient was transferred back to the general ward.
The barrier enclosure is an alternative physical barrier to large droplets or splatters during endoscopy, particularly if PPE is not available. However, the user must be mindful of the limitations that come with its use, including a potential for increased airborne particle exposure, if not properly utilized.
NOTES
1) Cleaning and Disinfecting Your Facility. Available from https://www.cdc.gov/coronavirus/2019-ncov/community/disinfecting-building-facility.html
Acknowledgments
Jin Long Chong for gifting us with the barrier enclosure box and Christopher Chia, endoscopist, for the procedure.
Fig.┬Ā1.
(A) Use of a barrier enclosure during endoscopic retrograde cholangiopancreatography (Simulated Photo). The endoscopist to the right of the patient, with the anesthetist at the head end. Circular openings in the barrier enclosure ŌĆō 3 openings on the proximal surface, to allow the anesthetist's arms and equipment (nasal cannula, suction tubing) to pass through; 1 on the endoscopist's side, through which the endoscope is placed; 2 on the side opposite to the endoscopist, through which an assistant may access the patient. (B) Dimensions of the barrier enclosure device. Length: 550 mm, Height: 600 mm, Width: 610 mm, Diameter of bigger circular openings: 190 mm.
References
1. British Society of Gastroenterology. Endoscopy activity and COVID-19: BSG and JAG guidance [internet]. London: bsg; 2020 Apr 3 [cited 2020 Jun 8]. Available from https://www.bsg.org.uk/covid-19-advice/endoscopy-activity-and-covid-19-bsg-and-jag-guidance/
2. Kearsley R. Intubation boxes for managing the airway in patients with COVID-19. Anaesthesia 2020; 75: 969.



3. Morgenstern J. Aerosols, Droplets, and Airborne Spread: Everything you could possibly want to know [internet]. First10EM; 2020 Apr 6 [updated 2020 Apr 6; cited 2020 Jun 8]. Available from https://first10em.com/aerosols-droplets-and-airborne-spread/

4. Canelli R, Connor CW, Gonzalez M, Nozari A, Ortega R. Barrier Enclosure during Endotracheal Intubation. N Engl J Med 2020; 382: 1957-8.











