 |
 |
| Korean J Anesthesiol > Volume 73(4); 2020 > Article |
|
Abstract
Background
In sugammadex-induced anaphylaxis, sugammadex and/or sugammadex-rocuronium complex have possible allergenic epitope.
Case
We report a case of sugammadex-induced anaphylaxis during general anesthesia in a 60-year-old male undergoing orthopedic hand surgery, manifesting as profound hypotension and urticaria. The timing of onset was closely associated with sugammadex administration. The patient recovered after extensive therapy including fluid, epinephrine, other vasopressors, steroid, and antihistamine administration. By intradermal skin test which was done at four weeks after anaphylaxis, we confirmed positive reactions to both sugammadex and sugammadex-rocuronium complex.
Since the introduction of sugammadex into clinical practice, several cases of sugammadex-induced anaphylaxis have been reported [1-3]. Sugammadex is a synthetic ╬│-cyclodextrin that can reverse neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery. Its usage was approved by the US Food and Drug Administration in 2015, which was later compared to its approval in Europe in 2008. Such delay in approval seemed to be partly due to lack of data regarding its adverse reactions including hypersensitivity. We have experienced sugammadex-induced anaphylaxis and confirmed that the patient was reactive to both sugammadex and sugammadex-rocuronium complex by the intradermal skin test. Only one case report has documented the similar reactivity shown in our case [4]. But, in the reports that tested the sugammadex-rocuronium complex, methods to make the complex were different from each other [2,4,5]. Therefore, we would like to consider the skin test method with case review. Written informed consent was obtained from the patient for publication of this case report and accompanying images.
This study was approved by St.Mary's Hospital, The Catholic University of Korea (SC19ZESE0137).
A 60-year-old male (weight: 64 kg, height: 159 cm) was scheduled for emergency incision, drainage, and flap coverage on soft tissue infection of the right hand. He had amputation of the right index finger at another hospital 26 years ago, and had middle finger amputation and flap coverage in our orthopedic department seven years ago. Both surgeries were performed under general anesthesia without exposure to sugammadex. He had hypertension that was not treated. He did not have allergy history.
After he entered the operating room with anxiety, blood pressure (BP) 220/118 mmHg, heart rate 55 beats per minute (bpm), and O2 saturation by pulse oximetry 97% at room air were measured. Because a long time operation was scheduled and the patient refused regional anesthesia, we decided to perform general anesthesia. Within 5 min after we injected midazolam 3 mg and nicardipine 0.5 mg, he became comfortable and his BP decreased to 155/99 mmHg. After preoxygenation, general anesthesia was induced with propofol 120 mg and succinylcholine 70 mg using rapid sequence technique with supplemental remifentanil infusion. Following endotracheal intubation, we maintained anesthesia with inhalational gas mixture of oxygen (FiO2 0.4)-air-desflurane (6 vol%), supplementary remifentanil infusion at a rate of 0.03ŌĆō0.07 ╬╝g/kg/min, and neuromuscular blocking agent rocuronium (EsmeronŌōć, MSD, the Netherlands) 40 mg. At 30 min after the operation, only a biopsy was performed and the operation was terminated because the malignancy was strongly suspected. Total anesthetic duration was 40 min. We discontinued the administration of all anesthetics. Sugammadex (Bridion┬«, MSD, the Netherlands) 200 mg was used to antagonize neuromuscular blockade at a train of four count of two monitored with a peripheral nerve stimulator (TOF watch┬«, Organon, Ireland). The patient started to breathe spontaneously while BP increased up to 207/119 mmHg concurrently that was treated with nicardipine 0.5 mg again. About 3 min after extubation while we prepared to transport the patient, his BP decreased suddenly to 78/38 mmHg. After 10 mg of ephedrine and 100 ╬╝g of phenylephrine injection to compensate suspicious nicardipine overdose, BP rose to 93/48 mmHg. He was transferred to the post anesthetic care unit (PACU).
During transport, he complained of itching sense at the buttock area. Just after arrival at the PACU, we found urticarial rashes on the neck, chest, and buttock. Soon, he became rapidly hypotensive (66/41 mmHg) and tachycardic (104ŌĆō105 bpm). In turn, he developed generalized urticaria over the whole body. An anaphylactic shock was strongly suspected on the basis of hemodynamic data and skin manifestation. We rapidly elevated his both legs, increased fluid infusion rate, and started infusion of epinephrine. We added norepinephrine to increase BP effectively. Also, methylprednisolone 125 mg, hydrocortisone 100 mg, and pheniramine 4 mg were given intravenously. Portable echocardiography showed no abnormal wall motion except dehydration. Arterial blood gas analysis was normal. After 30 min, his condition improved with a stable vital sign. After we explained anaphylaxis to the patient, he was transported to the intensive care unit for overnight observation. His serum mast cell tryptase level was 2.4 ╬╝g/L at 12 h and 5.8 ╬╝g/L at 36 h post-event (reference value: < 11.0 ╬╝g/L). His levels of immunoglobulins E, G, A, and M were normal.
After one month from anaphylaxis, he was scheduled for an elective operation on the right first and third metacarpal bone resection. His hypertension was treated with atenolol. The plan of regional anesthesia was denied by the patient. After informed consent, an intradermal skin test was performed under general anesthesia. We anesthetized the patient in the same manner as prior anesthesia, but we used vecuronium (instead of rocuronium) and anticholinesterase (neostigmine 1.5 mg) with anticholinergics (glycopyrrolate 0.4 mg).
For the intradermal test, we prepared dilutions of test drugs: nicardipine 1 mg/ml (diluted 1 : 100), rocuronium 10 mg/ml (diluted 1 : 100), and sugammadex 100 mg/ml (diluted 1 : 100, 1 : 1000). We also prepared a mixture of rocuronium and sugammadex to test the sugammadex-rocuronium complex. Sugammadex (1 : 500 dilution) was mixed with the same volume of rocuronium (1 : 50 dilution). Thus, the final dilutions of the two drugs were 1 : 1000 and 1 : 100, respectively. This complex is known to have very few free sugammadex molecules. We used histamine (0.01%) as positive control and normal saline as negative control. Intradermal testing was conducted according to standard protocol on the patientŌĆÖs volar forearm with a 25 G needle, resulting in a 5 mm wheal, 30 mm apart from each other. After 20 min, we evaluated the intradermal skin test results.
The skin tests were positive to 1 : 100 (20 ├Ś 10 mm wheal, 44 ├Ś 38 mm flare) and 1 : 1000 (14 ├Ś 9 mm wheal, 43 ├Ś 47 mm flare) dilutions of sugammadex. Sugammadex-rocuronium complex site also showed a positive reaction (24 ├Ś 10 mm wheal, 50 ├Ś 35 mm flare) (Fig. 1). Reactions to normal saline, nicardipine, and rocuronium were negative. There was no systemic allergic reaction during the test.
He received one more general anesthesia for vitrectomy due to retinal detachment. We avoided the use of sugammadex again. Currently he is on treatment of primary cancer of skin appendage, lung cancer, and bone metastasis.ŌĆā
Although the overall incidence of hypersensitivity seems to be low at 0.22% and the incidence of anaphylaxis is as low as 0.059% in subjects who receive sugammadex under general anesthesia [6], signs and symptoms of reported cases are very severe to anesthesiologists and patients [7]. Clinically, skin rash and hypotension were most commonly presented. The very strong probability of sugammadex-induced anaphylaxis in most cases is based on time correlations between drug administration and appearance of symptoms and signs. In our case, the use of nicardipine to control hypertension and successive hypotension made us hesitate to diagnose anaphylaxis shortly, because nicardipine and sugammadex were injected at almost the same time. Persistent hypotension and skin manifestation were very helpful for the diagnosis of anaphylaxis.
For diagnosis of anaphylaxis, plasma histamine and serum tryptase may be helpful [8,9]. For plasma histamine assay, sampling time is important because it is increased for 15ŌĆō60 min after the appearance of anaphylaxis. Also, special handling is required for sampling and manipulation, such as obtaining blood through wide-bore needle, keeping sample cold at all times, immediate centrifuging, and prompt freezing of the plasma [8]. On the other hand, serum and plasma tryptase levels are increased 15 minŌĆō3 h after the appearance of anaphylaxis and ordinary sampling way is required. Measurement of both plasma histamine and tryptase can increase the diagnostic accuracy. To our regret, the proper sampling time for detection of plasma histamine and serum tryptase had passed. Histamine assay was omitted and tryptase levels were normal in the sample taken late. It is true that an elevated acute serum tryptase is highly predictive of IgE-mediated anaphylaxis. However, normal tryptase level does not preclude anaphylaxis according to its sensitivity, specificity, positive predictive value, or negative predictive value [10].
The skin test is superior to the analysis of histamine and tryptase for the diagnosis of anaphylaxis. In the cases of sugammadex-induced anaphylaxis, concurrent positive reactions to sugammadex and sugammadex-rocuronium complex have been rarely reported [2,4]. In some cases including this report, the skin tests for sugammadex and sugammadex-rocuronium complex were performed at the same time from the beginning [4,5]. In other cases, the skin test for the sugammadex-rocuronium complex was performed later to find out the possible allergenic antigen after negative reaction to sugammadex [2,11]. Nakanishi et al. [4] mixed equal volumes of sugammadex 100 mg/ml and rocuronium 10 mg/ml and made a serial dilution (1 : 10, 1 : 100, and 1 : 1000), yielding the ratio of sugammadex to rocuronium in the mixture 2.8 : 1 on a molar basis. By skin prick test, their patient was positive to sugammadex and undiluted rocuronium-sugammadex mixture. The authors suggested that a larger amount of free sugammadex molecules in the mixture seemed to elicit a positive skin reaction. In another case, Sadleir et al. [5] mixed equal volumes of 1 : 500 sugammadex and 1 : 50 rocuronium to make sugammadex 1 : 1000, rocuronium 1 : 100, respectively, resulting in a final concentration of 0.1 mg/ml for each drug. In that way, they expected very few free sugammadex molecules present in the mixture because the ratio of sugammadex to rocuronium in the mixture was 1 : 3.6 on a molar basis. Their patient was reactive to sugammadex, but not reactive to sugammadex-rocuronium complex. The authors suggested that the allergenic epitope of sugammadex was occupied by the binding of two molecules or it might have undergone conformational change that it could not bind to IgE. Ho et al. [2] have mixed equal volumes of rocuronium at 1 : 500 dilution (0.02 mg/ml) with sugammadex 1 : 50 (2 mg/ml). Their patient had a negative reaction to sugammadex, but a positive reaction to the sugammadex-rocuronium complex and sugammadex-vecuronium complex. In our case, although we used the method described by Sadleir et al. [5], different results were obtained. Our patient had a positive reaction to both sugammadex and sugammadex-rocuronium complex, suggesting that both of sugammadex and sugammadex encapsulating rocuronium expressed possible antigenic epitope. Like the case of anaphylaxis to sugammadex showing that ╬│-cyclodextrin in sugammadex was the causative allergen by skin test [12], the unchanged part common to sugammadex and sugammadex-rocuronium complex might have acted as allergen. It is extremely rare for both sugammadex and sugammadex-rocuronium complex to be identified as allergens in one patient [4]. However, as shown in this case, the anaphylactic symptoms were not more severe than other cases caused by only sugammadex or only sugammadex-rocuronium complex.
The dose of sugammadex that caused anaphylaxis was varied from 0.75 to 4 mg/kg [1,11]. Although the administered dose per body weight varies from patient to patient, many anesthesiologists administered 200 mg of sugammadex in the patients regardless of body weight, resulting in various concentrations of free sugammadex [2-4]. The sugammadex dose we used in this case was 200 mg (about 3.13 mg/kg, body weight). This was more than the recommended dose which was 2 mg/kg of sugammadex at TOF 2/4. Thus, more free sugammadex molecules would have existed in the patientŌĆÖs blood at high levels, which might be involved in inducing anaphylaxis. There are two reports about sugammadex and hypersensitivity incidence in healthy non-anesthetized subjects. de Kam et al. [13] have reported that hypersensitivity or anaphylactic reaction to sugammadex is dose-dependent while Min et al. [14] have concluded that hypersensitivity incidence is similar across sugammadex doses. Although these two experimental conditions were different from clinical anesthesia, severe hypersensitivity reactions occurred clearly after a high dose of 16 mg/kg. In clinical situations, it is difficult to determine the correlation between sugammadex dose or concentration and anaphylaxis incidence based on the data obtained so far.
For skin test after anaphylaxis, we have to wait 4ŌĆō6 weeks because of temporary loss of cutaneous activity following anaphylaxis. Clinicians need a minimum of two weeks after anaphylaxis to perform skin test so that there is sufficient time to restore the cutaneous reactivity. However, inconclusive results might be possible [15].
To treat anaphylaxis, most physicians use adrenaline, steroids, antihistamines, and bronchodilators in case of bronchospasm [9,15]. Our first choice of drug was epinephrine, the first-line therapy in anaphylaxis guidelines. We had to replace volume by leg-up position and rapid fluid infusion, added norepinephrine, and administered steroids and antihistamines. Fortunately, no patient has died after sugammadex-induced anaphylaxis from all the case reports. Typically, clinical features of sugammadex anaphylaxis appeared when the patient was already extubated, being transferred to ward bed, or PACU [8]. This very busy time point is almost consistent with the report that symptoms are all expressed within 4 min [7]. Anesthesiologists should pay constant attention in blood pressure when using sugammadex because extreme hypotension is the first sign in most cases. It seems that there is no difference in the degree of symptom between anaphylaxis induced by sugammadex and that induced by the sugammadex-rocuronium complex [1-5,8].
A limitation of this case study is that a specific method to make the sugammadex-rocuronium complex for skin test is not established, yet. Also, the method of skin test varies from author to author.
Sugammadex-induced anaphylaxis is emerging as its usage is increased. Clinical presentation and timing of onset related to sugammadex administration are important clues for early diagnosis of sugammadex-induced anaphylaxis. After starting initial standard therapy for anaphylaxis, sampling for plasma histamine and serum tryptase evaluation within an appropriate time might be helpful. For precise diagnosis, it is important to perform a skin test for suspicious allergen, including sugammadex and sugammadex-rocuronium complex. However, the exact way to make inclusion complex is not established yet. Thus, when reporting this case of sugammadex anaphylaxis showing positive reactions to both sugammadex and sugammadex-rocuronium complex, simultaneous skin test for sugammadex and sugammadex-rocuronium complex would be helpful for further detection of an allergenic part.
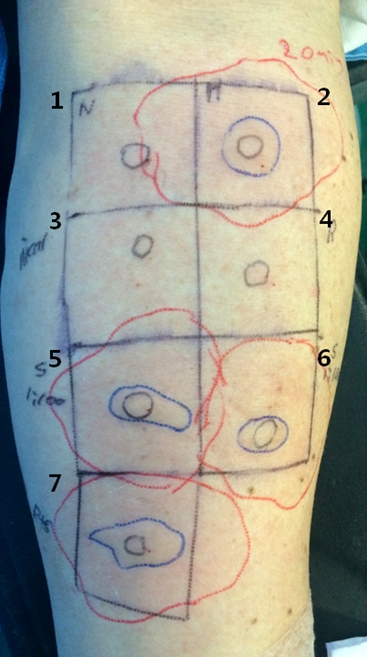
Fig.┬Ā1.
Intradermal skin test with suspicious allergen drugs. Sugammadex at both dilutions of 1 : 100 and 1 : 1000 showed positive reaction (5,6). The sugammadex-rocuronium complex also showed positive reaction (7). The red lines are the contour of flare and the blue lines are the contour of wheal. 1: normal saline as negative control, 2: histamine 0.01% as positive control, 3: nicardipine 1 : 100 dilution; 4: rocuronium 1 : 100 dilution, 5: sugammadex 1 : 100 dilution, 6: sugammadex 1 : 1000 dilution, 7: sugammadex-rocuronium complex.
References
1. OŌĆÖDonnell R, Hammond J, Soltanifar S. A confirmed case of sugammadex-induced anaphylaxis in a UK hospital. BMJ Case Rep 2017; 2017: bcr2017220197.



2. Ho G, Clarke RC, Sadleir PH, Platt PR. The first case report of anaphylaxis caused by the inclusion complex of rocuronium and sugammadex. A A Case Rep 2016; 7: 190-2.


3. Yoo JH, Kim SI, Ok SY, Park SY, Cho A, Han YM, et al. Suspected anaphylactic reaction associated with sugammadex: a case report. Korean J Anesthesiol 2016; 69: 413-6.




4. Nakanishi T, Ishida K, Utada K, Yamaguchi M, Matsumoto M. Anaphylaxis to sugammadex diagnosed by skin prick testing using both sugammadex and a sugammadex-rocuronium mixture. Anaesth Intensive Care 2016; 44: 122-4.

5. Sadleir PH, Russell T, Clarke RC, Maycock E, Platt PR. Intraoperative anaphylaxis to sugammadex and a protocol for intradermal skin testing. Anaesth Intensive Care 2014; 42: 93-6.


6. Miyazaki Y, Sunaga H, Kida K, Hobo S, Inoue N, Muto M, et al. Incidence of anaphylaxis associated with sugammadex. Anesth Analg 2018; 126: 1505-8.


7. Tsur A, Kalansky A. Hypersensitivity associated with sugammadex administration: a systematic review. Anaesthesia 2014; 69: 1251-7.


8. Takazawa T, Mitsuhata H, Mertes PM. Sugammadex and rocuroniumŌĆæinduced anaphylaxis. J Anesth 2016; 30: 290-7.



9. Hsu Blatman KS, Hepner DL. Current knowledge and management of hypersensitivity to perioperative drugs and radiocontrast media. J Allergy Clin Immunol Pract 2017; 5: 587-92.


10. Krishna MT, York M, Chin T, Gnanakumaran G, Heslegrave J, Derbridge C, et al. Multi-centre retrospective analysis of anaphylaxis during general anaesthesia in the United Kingdom: aetiology and diagnostic performance of acute serum tryptase. Clin Exp Immunol 2014; 178: 399-404.



11. Okuno A, Matsuki Y, Tabata M, Shigemi K. A suspected case of coronary vasospasm induced by anaphylactic shock caused by rocuronium-sugammadex complex. J Clin Anesth 2018; 48: 7.


12. Hotta E, Tamagawa-Mineoka R, Masuda K, Taura M, Nakagawa Y, Kanehisa FA, et al. Anaphylaxis caused by ╬│-cyclodextrin in sugammadex. Allergol Int 2016; 65: 356-8.


13. de Kam PJ, Nolte H, Good S, Yunan M, Williams-Herman DE, Burggraaf J, et al. Sugammadex hypersensitivity and underlying mechanisms: a randomised study of healthy non-anaesthetised volunteers. Br J Anaesth 2018; 121: 758-67.











