EC50 and EC95 of remifentanil to prevent rocuronium-induced withdrawal movements in children
Article information
Abstract
Background
Intravenous administration of rocuronium induces intense pain in most patients (60-100%). This could be harmful during anesthesia induction because of the unintended reflex movement of an unconscious patient in response to the pain. Previous studies have reported that remifentanil effectively reduces rocuronium-induced pain and withdrawal movements. This study was designed to evaluate the EC50 and EC95 of remifentanil to prevent withdrawal movements in children.
Methods
We enrolled a total of 171 pediatric patients scheduled for general anesthesia in this study. Remifentanil was administrated by target-controlled infusion. Effect-site target concentrations ranged from 0.5 to 3.0 ng/ml. At each concentration, experiments were repeated in 10-20 patients. Propofol 2 mg/kg and rocuronium 0.9 mg/kg were administrated after equilibration of plasma and effect-site target remifentanil concentration. The withdrawal movements were graded on a 4-point scale. The EC50 and EC95 of remifentanil to prevent rocuronium-induced withdrawal movements were determined by using a logistic regression model.
Results
The logistic regression model showed that the probability of preventing rocuronium-induced withdrawal movement was as follows: exp (-3.49 + 2.07 × remifentanil concentration) / (1 + exp [-3.49 + 2.07 × remifentanil concentration]). EC50 and EC95 were 1.69 ng/ml (95% confidence intervals [CIs], 1.42-1.87) and 3.11 ng/ml (95% CIs, 2.79-3.72), respectively.
Conclusions
Administration of remifentanil at an effect-site target concentration of 3.1 ng/ml could effectively prevent rocuronium-induced withdrawal movements.
Introduction
Intravenous administration of rocuronium induces intense pain in most patients (60-100%) [1,2,3]. This pain can elicit either reflex withdrawal movements of the hand and arm where the injection site is located or generalized movements of the body in unconscious patients. During anesthesia induction, these withdrawal movements may cause dislocation of intravenous catheters, difficulties in administration of additional drugs, and subsequent risk of cardiovascular activation [4]. This is especially harmful to young children because of difficulties in rapid insertion of a catheter.
Remifentanil is a synthetic and esterase-metabolized opioid. It has a rapid onset and an ultra-short duration of action with a stable, short context-sensitive half-time [5]. Many studies have reported that remifentanil effectively reduces rocuronium-induced pain and withdrawal movements [6,7,8,9]. There have recently been studies on the EC50 and EC95 of remifentanil to prevent withdrawal movements in adults [4,10] and children [10] using the Dixon up-and-down method and by probit analysis. However, their clinical applicability may be limited because EC50 and EC95 reported in previous studies have very wide confidence intervals. This study was designed to evaluate the clinically useful EC50 and EC95 of remifentanil to prevent withdrawal movements in a large number of pediatric patients and by logistic regression analysis.
Materials and Methods
After obtaining approval from the Institutional Review Board of our hospital and written informed consent from the patients, 171 pediatric patients aged 5-12 years who were at American Society of Anesthesiology physical status I or II were enrolled. Patients with history of neurological deficits, allergies to anesthetic medications, asthma, or poor venous access, were excluded. Also, patients with severe separation anxiety who could not be delivered into the operating room without the administration of a hypnotic drug were excluded. None of the patients were premedicated with hypnotic or sedative medications before anesthesia. A 22-gauge intravenous cannula was inserted into the dorsum of the hand without subcutaneous lidocaine infiltration in the ward about 2 hours before the induction of anesthesia. Dextrose-saline solution was chosen as the intravenous fluid. On arrival at the operating room, each patient's electrocardiogram, pulse oximetric measurements, and non-invasive blood pressure were monitored, and a mask delivering 5 L/min of O2 was loosely applied to the face. Remifentanil was administrated by target-controlled infusion via a syringe pump (Pilot anesthesia 2, Fresenius vial, France) driven by STELPUMP (Ver. 1.07). The pharmacokinetics model proposed by Minto et al. [11] was used. Effect-site target concentrations used in this experiment ranged from 0.5 to 3.0 ng/ml. Patients were divided into 10 groups according to the effect-site target remifentanil concentrations (ng/ml): the remifentanil 0.5, 1.0, 1.5, 1.8, 2.0, 2.2, 2.4, 2.6, 2.8, and 3.0 groups. At each concentration, experiments were repeated in 10-20 pediatric patients.
After equilibration of plasma and effect-site target remifentanil concentrations, propofol 2 mg/kg was administrated. During administration of remifentanil and propofol, the assistants held the patients by the arms to keep them steady if needed. Immediately after loss of consciousness and the eyelash reflex, mask ventilation was started with O2 at 5 L/min, and 1% rocuronium was administrated at 0.9 mg/kg over 5 seconds into a port connected directly to the i.v. cannula. Withdrawal movements were observed by the same blinded investigator using a 4-point scale: 0 = no movement; 1 = movement limited to the wrist; 2 = movement limited to the arm; and 3 = generalized movement [2,6]. A grade of 2 or more was considered significant movements. The order of experiments was randomly selected, and the anesthesiologist who graded withdrawal movements was blinded to the concentrations of remifentanil used. After grading withdrawal movements, anesthesia was induced with sevoflurane 3-5 vol% and O2 100%, and tracheal intubation was performed 90 seconds later. Anesthesia was maintained with sovoflurane 1-5 vol% and 50% nitrous oxide in oxygen. Blood pressure and heart rate were recorded upon arriving at the operation room, after reaching the effect-site target concentration of remifentanil, and immediately after intubation.
Statistical analysis
Statistical analyses were performed using SPSS 18.0 (SPSS, Chicago, IL, USA). The EC50 and EC95 of remifentanil to prevent rocuronium-induced withdrawal movements were determined by a logistic regression model. The size of the samples was 5 times as large as that of a study done by using Dixon's method [12]. In previous studies [4,10] and our preliminary study, the predicted values of EC20-95 ranged from 1.0 to 3.0 ng/ml. On the basis of these results, remifentanil concentration was determined as 0.5 to 3.0 ng/ml in our study.
The significance of regression coefficients was verified by the t test. The fitness for model was verified by the Pearson goodness-of-fit test. Concentration response curve was plotted using the equation obtained from the logistic regression model. Hemodynamic data were analyzed by repeated measured ANOVA, and Tukey was used for post hoc multiple comparisons. Values were expressed as mean ± SD, mean (95% confidence intervals [CIs]), or number of patients. A P value of < 0.05 was considered statistically significant.
Results
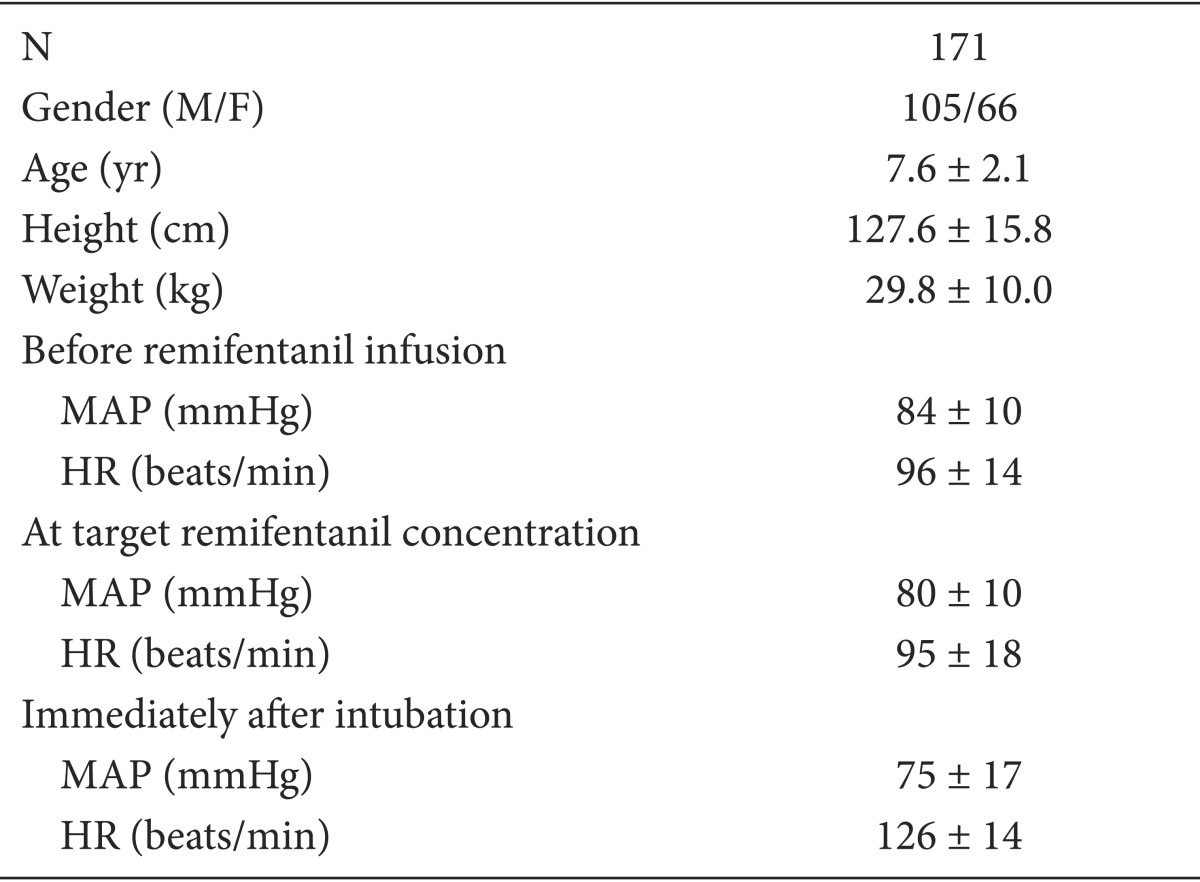
The demographic and hemodynamic data are shown in Table 1. There was no case of significant hypotension, bradycardia, or O2 desaturation during remifentanil infusion. Blood pressure showed a decreasing tendency in the experiments. The remifentanil 1.5 group showed a higher mean blood pressure than the remifentanil 3.0 group (P = 0.047). Heart rate increased in all groups immediately after intubation, but there were no significant differences between the 10 groups (Fig. 1). Since the range of the hemodynamic data was very wide (Table 1), a larger number of patients is needed to evaluate the exact hemodynamic effect of various remifentanil concentrations.

Heart rate and mean arterial pressure according to remifentanil concentrations. The remifentanil 1.5 group shows a higher mean blood pressure than the remifentanil 3.0 group (P = 0.047). Heart rate increased in all groups immediately after intubation, but there were no significant differences between the 10 groups.
To estimate the probability of preventing rocuronium-induced withdrawal movement (no response, grade of withdrawal movements 0 or 1) of various remifentanil concentrations, we fitted the experimental data to the logistic regression model. The Pearson goodness-of-fit test showed that our data fitted well to this model (P = 0.41). The logistic regression formula is as follows:

where
The regression coefficients were statistically significant (P = 0.00). EC50 was 1.69 (95% CIs, 1.42-1.87). EC95 was 3.11 (95% CIs, 2.79-3.72) (Table 2, Fig. 2).
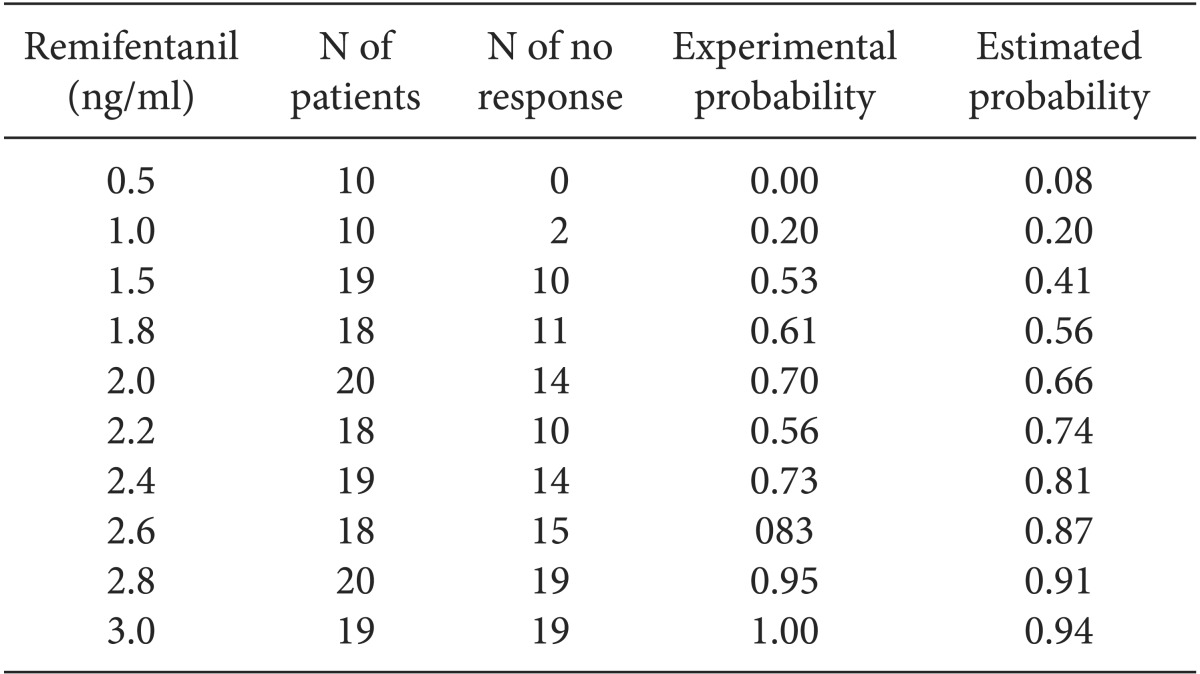
The Experimental and Estimated Probability of Preventing Rocuronium-induced Withdrawal Movements according to Effect-site Target Concentrations of Remifentanil
Discussion
This study aimed to determine the EC50 and EC95 of remifentanil to prevent rocuronium induced withdrawal movements. Rocuronium-induced withdrawal movements are known to be associated with a severe burning pain lasting for approximately 10-20 seconds [1]. Although the pathophysiological mechanism of the pain is unclear, direct activation of the c-nociceptor by the release of local mediators has been suggested [13]. It may also be associated with the osmolality and pH of drugs [14]. However, Tuncali et al. [15] have documented that dilution of rocuronium without changes in pH or osmolality eliminates rocuronium injection pain. The algogenic effect of aminosteroidal neuromuscular blockers could be prominent because rocuronium is a less potent neuromuscular blocking drug that needs the higher dose or concentration in clinical use [13]. Rocuronium injection pain is reportedly more common in pediatric patients because small vessels require a long time to wash out [7,10].
Numerous drugs have been studied to prevent rocuronium injection pain. Lidocaine [16], bicarbonate [17], esmolol [18], ondansetron [19], epedrine [20], and opioids [6,7,8] are known to be effective. Kim et al. [16] have demonstrated that the lidocaine occlusion method is more effective in adults and that addition of sodium bicarbonate is more effective in children. Many studies have indicated that pretreatment with opioids could prevent rocuronium injection pain effectively [6,7,8]. Remifentanil seems to be suitable especially for short-duration pain control on the basis of its pharmacokinetic profile [5]. While application of a pneumatic tourniquet is useful for drugs with local anesthetic properties, including lidocaine [16] and ondansetron [19], centrally acting drugs, like opioids, require time to reach equilibrium at the effect site. Remifentanil is known to act on the central opioid receptor rather than the peripheral opioid receptor [21]. A time interval of 90 seconds between injections of remifentanil and rocuronium is recommended for a maximal effect [21]. Remifentnil is known to have a dose-dependent effect. Kim et al. [7] reported that remifentanil 1 µg/kg was more effective in preventing rocuronium induced withdrawal movements than remifentanil 0.5 µg/kg. A few studies have reported the EC50 and EC95 of remifentanil to prevent rocuronium withdrawal movements in children [10] and adults [4,10] using the Dixon's up-and down method and by probit analysis. These studies were originally designed for Dixon's up-and down method that was suitable for calculating EC50. However, the number of patients seems to be too small for probit analysis in calculating for EC95. The resulting confidence interval was too wide for clinical use. The EC95 of remifentanil needs to be definitely established in order to be used in clinical practice. EC95 can be calculated with a small number of patients by using biased-coin design up-and-down method [22]. However, this method was designed to repeat experiments nearly up to target concentration. Thus, the concentration-response curve covering the wide range of concentrations may be hard to plot. By applying a logistic regression model and increasing the number of patients, we were able to reduce 95% confidence intervals to one-tenth of the value reported by a study by using Dixon's up-and-down method [4] and plot the concentration-response curve with acceptable confidence intervals. A logistic regression model is used in cases where dependent variables are discrete data. Through logistic transformation, discrete dependent variables are converted to continuous variables. As independent variables increase, the dependent variables converge to 1 [23].
The results of this study are subject to at least 2 limitations. First, this study was inadequate to evaluate the hemodynamic effect of increasing remifentanil concentration. Regardless of remifentanil concentrations (0.5-3.0 ng/ml) used in this study, heart rate increased immediately after intubation without significant differences between the individual groups with different remifentanil concentrations. The range of hemodynamic data was too wide to find any differences between the individual groups with different remifentanil concentrations. To evaluate hemodynamic response to increasing remifentanil concentrations, corresponding increases in the number of patients are required. Estimated EC95 for preventing rocuronium-induced withdrawal response is 3.11 ng/ml. This value is beyond the range of concentrations used by our study. Although it is uncertain whether remifentanil has a hemodynamic effect at a concentration of 3.11 ng/ml, it is inconceivable that remifentanil led to hemodynamic disturbance. An effect-site target remifentanil concentration of 3.0 ng/ml was unable to blunt the hemodynamic response of intubation. A much higher concentration of remifentanil is needed to simultaneously prevent rocuronium-induced withdrawal movements and hemodynamic response to intubation. Park et al. [24] reported that an effect-site target remifentanil concentration of 7.5 ng/ml improved hemodynamic stability after insertion of laryngeal mask airway [22]. Further studies are required to confirm our results. Second, Minto's pharmacokinetics model was used in this study. Minto's model was established on the basis of adult pharmacokinetic data [11]. When adult pharmacokinetics model is used in children, remifentanil blood concentration could be higher because the clearance is underpredicted [25]. Because there is a report that it is not satisfactory to use the adult pharmacokinetic model for children under 5 years [26], we limited the patient's age to be above 5 years in this study. However, Ross et al. [27] have shown that because both clearance and volume of distribution were inversely related to age, children aged >2 years have similar pharmacokinetic profiles as adults. Jeleazcov et al.[28] reported that Keo., the parameter to be vital for the prediction of effect site concentration in TCI system was similar to the values reported by Minto in children > 1 year. Although many studies of children have used Minto's pharmacokinetic model [10,24,29,30], establishment of pediatric pharmacokinetic models requires more accurate studies.
In summary, to prevent rocuronium-induced withdrawal movements in children, the EC50 of remifentanil was 1.69 ng/ml (95% CIs, 1.42-1.87), and EC95 of remifentanil was 3.11 ng/ml (95% CIs, 2.79-3.72). Administration of an effect-site target remifentanil concentration of 3.1 ng/ml could effectively prevent rocuronium-induced withdrawal movements in pediatric patients.