|
 |
|
|
Abstract
Background
As the number of elder patients grows, spinal anesthesia for such patients are increasing significantly. Any effort is needed to use the least anesthetic drug for maintaining the anesthesia while avoiding hazards of cardio-pulmonary complications.
Methods
American Society of Anesthesiologists physical status classification I and II, Forty five elderly patients (≥ 60 years) who received transurethral resection of the prostate or transurethral resection of the bladder tumor were allocated randomly into three treatment groups. The DMT 0.5 group was designed as with dexmedetomidine 0.5 µg/kg while the DMT 1.0 group has a 1 µg/kg intravenous injection over 10 min before anesthetic induction. The Control group was designed to get a normal saline. Each group was compared regarding the maximum sensory block level, extension of anesthesia, degree of motor block, level of sedation, VAS score and complications.
Results
There were no significant differences among the 3 treatment groups regarding the maximum level of sensory block and motor block. However, the duration of sensory block was significantly longer in DMT 1.0 group than in the control group (P = 0.045). Both DMT 1.0 group (median = 3, range = 2-6) and DMT 0.5 group (median = 3, range = 1-6) showed a mean value of 3-4 Ramsay sedation score, which resulted in more excessive sedation and significantly greater incidence of bradycardia compared to the control group. No complications such as hypotension, nausea, tremor, and hypoxia were found during this investigation.
When implementing a spinal anesthesia, a rapid change of blood pressure might occur. This could cause serious issues for elderly patients with a possible concomitant cardiovascular disease [1]. Therefore, before implementing spinal anesthesia, we can prevent a sudden hypotension according to the expansion of spinal anesthesia range due to injecting a sufficient amount of fluid and inotropics such as Ephedrine or Phenylephrine. However, there are also many elderly patients who have a medical history of hypertension, diabetes or transient ischemic accident. As a result, if such patients are anesthesized with more than T4 dermatome, they are more vulnerable to cardiovascular changes and their health status could be more likely irreversibly damaged by it.
As the population of elderly people increases, the number of elderly patients who get a surgery with spinal anesthesia is increasing accordingly. Examples for such surgeries which need spinal anesthesia are transurethral resection of the prostate or transurethral resection of the bladder tumor, hip fracture surgeries or total knee replacement arthroplasty. Therefore, it is important to implement spinal anesthesia with the use of a small amount of anesthetic medicine. In this way, we can get proper anesthesia level and reduce the effect on the cardiovascular system. However, a small amount of anesthetic medicine could lead to a shorter maintenance time as it is necessary for surgeries. Due to an unexpected prolonged surgery patients could feel pain on the operation region, and an unwanted general anesthesia could be necessary during this surgery.
Ebert et al. [2] demonstrated the time to sensory regressions of two dermatomes after ropivacaine spinal anesthesia increased in patients where dexmedetomidine was used (249 ± 56 min) compared to the control group (195 ± 53 min). Also, Hong et al. [3] found out dexmedetomidine can certainly increase the anesthesia time. It was detected during an investigation with elderly patients who got small amounts (6 mg) of Bupivacaine injected. Likely dexmedetomidine can prolong a spinal anesthesia and could so a larger dose of local anesthetics. As an additional benefit there is no risk of paradoxical excitation related to Midazolam.
In our research, spinal anesthesia was implemented to patients who got a transurethral resection of the prostate or a transurethral resection of the bladder tumor by using 6 mg of bupivacaine similar to the research of Hong. We built 3 treatment groups with the aim to find the proper dosage which could lead to a sedation of elderly patients with less side effects and an increased anesthesia effect. The control group was designed with a normal saline injection. The second group was designed to inject 0.5 µg/kg dexmedetomidine, and the third group was designed to inject 1 µg/kg dexmedetomidine.
We randomly selected 45 male patients with an age 60 years or older who underwent a transurethral resection of the prostate or transurethral resection of the bladder tumor. In general, a surgery took no longer than two hours. These patients there surgery in our hospital and their physical status was measured according to the American Society of Anesthesiologists (ASA) I, II. For this investigation we got a hospital ethics committee authorization. An informed consent form of patients was given beforehand.
Out of the patients who were planned to get an urology surgery in our hospital, following patients were excluded from the subject of investigation: patients with a ASA physical status 3 or higher, patients with coagulopathy or bleeding diathesis, with increased intracranial pressure, with sepsis, with hypersensitivity reaction to local anesthetics, with a psychologically unstable status with preexisting neurological deficits and with intake of narcotic analgesics or sedatives.
Forty-five patients were randomly divided into 3 groups. The control group was designed with 20 ml normal saline as injection. The second and the third group were designed with a fluid injection for 10 minutes. This fluid was a mixture of dexmedetomidine (Precedex, Hospira Inc., Lake Forest, IL, USA) 200 µg/2 ml and 18 ml normal saline. The second group (DMT 0.5 Group), patients got a fluid injection with 0.5 µg/kg and the third group (DMT 1.0 group) patients got an injection with 1 µg/kg. Initial patients got 500 ml lactated Ringer's solution starting with the entrance in the operating room until the time before spinal anesthesia was implemented without any other premedication. After the operating room entrance, electorocardiography, noninvasive blood pressure, pulse oximetry and basal vital sign were measured. Thereafter dexmedetomidine or normal saline were injected for 10 minutes. Five minutes after the injection, spinal anesthesia was implemented at the level of L3-L4. After confirming the effusion of the cerebrospinal fluid by puncturing with 25 gauge Quinck needle bupivacaine 6 mg were injected.
We confirmed disappearance of cold feeling by using alcohol sponge and pinprick sensation by using a needle. We recorded each of the degree of maximum sensory blockade. Also, by using Modified Bromage scale (Bromage 0: no paralysis, Bromage 1: unable to raise extended leg, Bromage 2: unable to flex knee, Bromage 3: unable to flex ankle), we confirmed the extent of motor blockade. During surgery performance we measured every 10 minutes and recorded the recovery time of sensory block to two dermatome regression from the maximum level and motor blockade recovered to bromage 1. No maintenance dose of dexmedetomidine was added and the experiment was continued by using a double blind study design. It means the observer of patients couldn't recognize them. The Ramsay sedation score was measured (1 = anxious and agitated, 2 = cooperative and tranquil, 3 = drowsy but responsive to verbal command, 4 = asleep but brisk responsive to a glabellar tap, 5 = asleep with a sluggish response to tactile stimulation, 6 = asleep and no response) in the operating room and recovery room on a 10 minute basis and observed the aspects of change during different time periods. We defined excessive sedation with a Ramsay sedation score 5/6. In the it happened, we confirmed the spontaneous breathing of patient. It was done carefully so that airway could be gained immediately if a hypoxia (oxygen saturation < 90%) occured.
We recorded complications caused by spinal anesthesia and dexmedetomidine such as bradycardia, hypotension, nausea, vomiting, shivering and hypoxia. We injected 0.5 mg of atropine intravenously if a bradycardia (heart rate < 50 /min) occured and injected 5 mg of ephedrine intravenously if a hypotension (systolic blood pressure < 90 mmHg, or average arterial blood pressure lower than 20% of standard) occured. After surgery, we watched patients in the recovery room until sensory blockade dropped to T12. We confirmed the patients degree of pain by using a visual analogue scale 1, 4, 12 and 24 hours postoperative. We also recorded the first time that the patients asked for the analgesia and the number of those requests over 24 hours.
Before this research a pilot study on with 10 patients for each of 3 groups was conducted. We used the average time in which the sensory block dropped to two dermatome (control group: 99 min, DMT 0.5 Group: 111 min, DMT 1.0 group: 130 min) for the calculation of patient numbers. We set alpha value as 0.05, beta value as 0.2. As a result we needed 45 samples in total (G-power 3.0.10). We used SPSS 13.0 (SPSS Inc., Chicago, IL, USA), and every measured value was marked as average ± standard deviation. For the comparison of age, weight, height, hemodynamic value, sensory and motor blockade of the three groups, we used an one-way analysis of variance, ANOVA. For the nonparametric variables of those three groups we used the Kruskal-Wallis test. For side-effects during the operation and the use of inotropics the analysis was done with the chi-square test. We decided P < 0.05 as statistically significant.
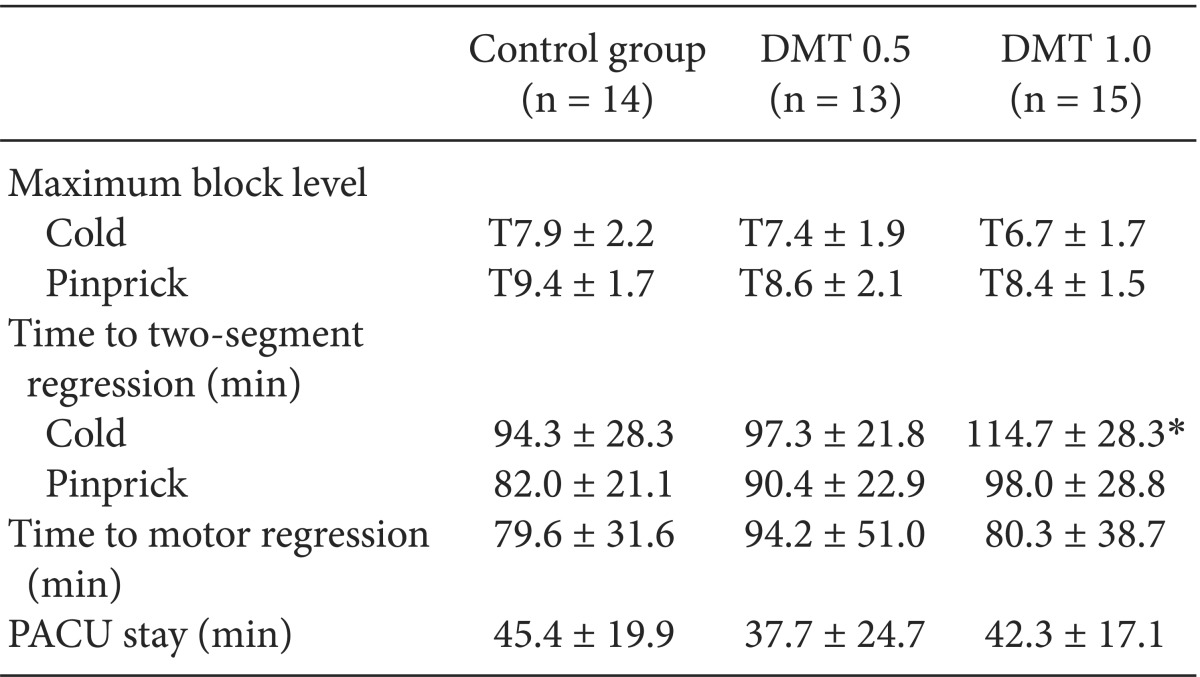
There were no significant differences in age, height and weight among the three groups (Table 1). We implemented spinal anesthesia in all patients. However, one patient from the control group, and 2 patients from the DMT 0.5 Group were excluded. This was due to we tried again the anesthesia or switched to general anesthesia because the anesthetic level was insufficient.
In both methods, the disappearance of cold and pinprick, the extent of maximum sensory blockade showed no significant differences among the groups. We found the disappearance of cold due to the anesthetic effect (time for sensory regression of two dermatomes) was 20 minutes (P = 0.045) longer in the DMT 1.0 group then in the control group. Even without statistical meaning the pinprick test showed also a longer anesthetic effect for about 16 minutes in the DMT 1.0 group then in the control group. The time it took for the motor blockade to recover was similar for all groups, and the time patients stayed in the recovery room showed also no statistical difference (Table 2). Almost all patients showed a 0-1 degree of pain using the Visual Analogue Scale (VAS) 1 ,4, 12, 24 hours postoperative, which means there was almost no pain. Therefore, the time that patients asked for the analgesia and the number of their requests for 24 hours was similar among the groups. This means barely no patient did complain pain. In a small number of cases patients asked for analgesia because they felt back pain or something uncomfortable on their foley catheter. However it did not affect the stats. There was no patient who showed excessive sedation in the control group. To show excessive sedation means that patients respond slowly to the tactile stimulation (Ramsay sedation score = 5), or a patients can't respond to the stimulus (Ramsay sedation score = 6). In the DMT 0.5 Group, 5 patients had score 5 and 1 patient had score 6. In the DMT 1.0 group, 5 patients had score 7 and 2 patients had score 6, which was significantly more than in the control group. In both DMT groups, sedation score was the deepest around the 20-minute period and it decreased thereafter (Fig. 1).
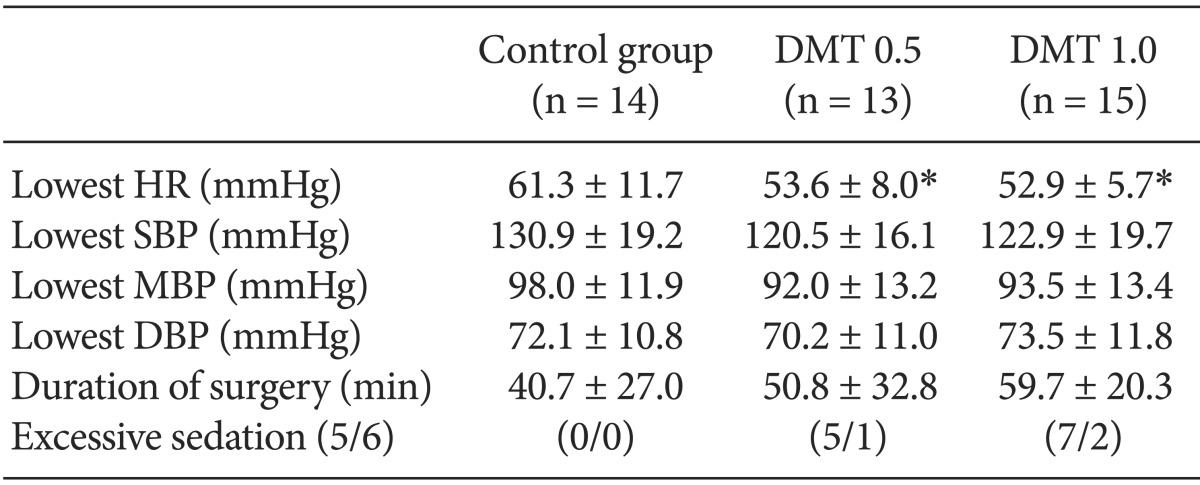
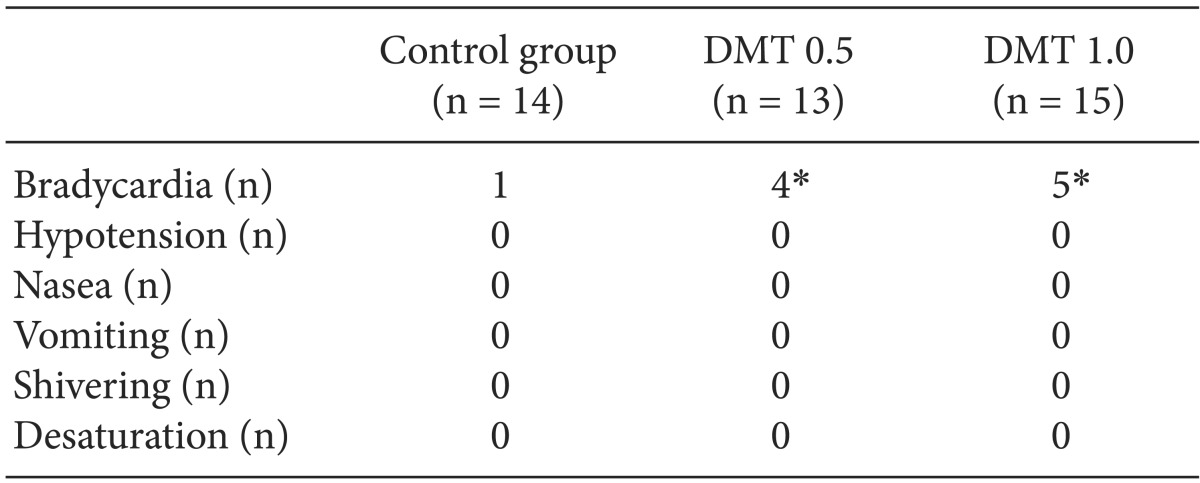
Vital sign were measured from the moment normal saline or dexmedetomidine were injected to the moment when the surgery was finished. The lowest heart rate was significantly different between the control group and the other groups. In the control group it was 61.3 ± 11.7 beats per minute whereas in the DMT 0.5 Group it was 53.6 ± 8.0 beats per minute (P = 0.025) In the DMT 1.0 group it was 52.9 ± 5.7 beats per minute (P = 0.019). The lowest heart rate was significantly lower in the both DMT groups. There was no significant difference among the groups considering the lowest hemodynamic values during the operation (Table 3). In DMT 0.5 Group and DMT 1.0 group the number of patients who suffered bradycardia (heart rate < 50 /min) was significantly larger than in the control group, but there was no difference between the two DMT groups (Table 4). No side effects such as hypotension, nausea, vomiting, shivering and hypoxia occurred.
If we inject α2-adrenergic receptor agonists such as Clonidine and dexmedetomidine with local anesthetic intrathecal, it can strengthen the effect of the local anesthetic. So it reduces the amount of local anesthetic which were needed and can extend the time of sensory blockade and motor blockade. There are several researches regarding this [4,5,6]. Through these, usefulness and stability of dexmedetomidine are confirmed [7]. Dexmedetomidine is a α2-adrenergic receptor agonist which is 8 times more selective than Clonidine [8]. Dexmedetomidine gives direct anesthetic effect by affecting brain and spinal neural tube. It also acts as a vasoconstrictor [9]. Many researches proved intravenous dexmedetomidine decreases the amount of inhalation anesthetics and narcotic analgesics which are needed during general anesthesia [10]. It also interacts with local anesthetics, decreases the needed amount and increases the effect. Also it is known with implementing a spinal anesthesia besides epidural anesthesia and local anesthesia intravenous dexmedetomidine increases the sensory blockade of anesthesia, it extends the effect of bupivacaine. Also the analgesia request time for patients for the first time after the surgery will be delayed [11]. There are also many researches regarding this.
In their research Hong et al. [3] implemented spinal anesthesia by using 6 mg of bupivacaine on 51 elderly patients. They observed a control group where patients got normal saline injection and another group where 1 µg/kg dexmedetomidine were injected. The extent of maximum sensory anesthesia and motor blockade had no difference. The time it took for the extent of anesthesia to drop down to two dermatome was prolonged in the group injected with dexmedetomidine in both cases of cold and pinprick sensation. The lasting time of motor blockade had a significant difference. In our study we want to see if we can get similar effects with fewer side effects by using smaller dosages. Therefore, in this research, we made two experimental groups (0.5 µg/kg, 1 µg/kg) and a placebo control group with elderly male patients and tried to find out the extent of anesthesia. We wanted to find out the proper dosage which could increase the effect of anesthesia and sedation with less side effects through this prospective research.
The heart rate of patients in both DMT groups dropped 8-9 beats per minute compared to that of the control group. The number of times we used atropine because bradycardia occurred was 1 in the control group, whereas 4 in the DMT 0.5 Group and 5 in the DMT 1.0 group. We expected bradycardia will occur more in the DMT 1.0 group, but there was no statistical difference. For DMT 0.5 Group, it was 53.6 ± 8.0 beats per minute, and for the DMT 1.0 group 52.9 ± 5.7 beats per minute. Also, other hemodynamic values had no difference. So we can conclude the control group and the DMT groups have statistically significant differences in terms of heart rate decline. However between the two DMT groups there is no difference regarding the heart rate and other hemodynamic values.
If we compare the control group with the DMT 0.5 Group in both cases of cold and pinprick sensation no statistical differences in the extent of anesthesia and in the effect of anesthesia extension were shown. However, compared with the control group, the time for the sensory blockade to drop down to two dermatome was 20 minutes longer for patients in the DMT 1.0 group. Also, without statistical difference, there was 17 minute difference in the prolonged time of anesthesia between the two DMT groups. The sensory blockade checked through the use of pinprick sensation showed similar results. Therefore, it is true that there was an effect of anesthesia extension in the DMT 1.0 group compared to the control group, but compared to the DMT 0.5 Group there are no statistical differences in the hemodynamic change such as bradycardia. The DMT 0.5 Group showed no meaningful differences in the effect of anesthesia extension compared with the control group. We need to reconsider that the dosage of the DMT 0.5 Group has no worth in the use for elderly patients because it showed similar frequencies of bradycardia compared to the DMT 1.0 group. Previous researches done by Kaya et al. [11] also showed the recovery time for motor blockades has no statistical meaning among the control group to the DMT groups.
If we use midazolam for sedation, some patients can show paradoxical excitement such as confusion, restlessness, being unable to follow directions and showing intermittent violent behaviors. There is no clear reason behind this, but it is known that it can affect 10.2% of elderly patients aged 65 years or older [12]. If we increase the dosage of dexmedetomidine 0.2-1.0 µg/kg/h, the extent of sedation deepens [13]. But unlike other sedatives, it can make patients easily arouse and more cooperative [14]. Cheung and Erdurmus et al. [15,16] evaluated the sedation effect of dexmedetomidine by injecting it until patients reached 3 or 4 point on Ramsay sedation score and with confirming the patient's comfortableness and the satisfaction of the operating surgeon. In this research we maintained in the DMT groups 3-4 point of Ramsay sedation score on average during the operation, which means that we gave them comfort. However, anesthetists' caution is needed because there were 1 patient in the DMT 0.5 Group and 2 in the DMT 1.0 group which showed excessive sedation by not reacting to tactile response. Even without additional time for the dexmedetomidine injection the operation time was 40 to 60 minutes. No maintenance dose was given during the surgery. Our conclusion was there is no time difference among the three groups regarding the stay in the recovery room. Expected side effects due to spinal anesthesia and dexmedetomidine such as hypotension, nausea, vomiting and hypoxia did not occur. Therefore, we couldn't compare the shivering preventing effect of dexmedetomidine which is caused by tonic vasoconstriction in the research of Usta et al. [17,18].
As a conclusion for both groups with intravenous dexmedetomidine, even a little amount of local anesthetic agent showed an extension of spinal anesthesia. However, anesthesiologists should be aware of possible bradycardias and excessive sedation which can occur due to dexmedetomidine. The DMT 0.5 group showed similar incidence of bradycardia to the DMT 1.0 group while the DMT 1.0 group was superior in aspect of the prolonged duration of sensory block compared to the DMT 0.5 group. We believe that more research is needed to identify the optimal dose of dexmedetomidine in both aspects of sedation and extension of spinal anesthesia.
References
2. Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 2000; 93: 382-394. PMID: 10910487.


3. Hong JY, Kim WO, Yoon Y, Choi Y, Kim SH, Kil HK. Effects of intravenous dexmedetomidine on low-dose bupivacaine spinal anaesthesia in elderly patients. Acta Anaesthesiol Scand 2012; 56: 382-387. PMID: 22220945.


4. Strebel S, Gurzeler JA, Schneider MC, Aeschbach A, Kindler CH. Small-dose intrathecal clonidine and isobaric bupivacaine for orthopedic surgery: a dose-response study. Anesth Analg 2004; 99: 1231-1238. PMID: 15385382.


5. Dobrydnjov I, Axelsson K, Thörn SE, Matthiesen P, Klockhoff H, Holmström B, et al. Clonidine combined with small-dose bupivacaine during spinal anesthesia for inguinal herniorrhaphy: a randomized double-blinded study. Anesth Analg 2003; 96: 1496-1503. PMID: 12707157.


6. Dobrydnjov I, Axelsson K, Samarütel J, Holmström B. Postoperative pain relief following intrathecal bupivacaine combined with intrathecal or oral clonidine. Acta Anaesthesiol Scand 2002; 46: 806-814. PMID: 12139535.


7. Gordh T Jr, Post C, Olsson Y. Evaluation of the toxicity of subarachnoid clonidine, guanfacine, and a substance P-antagonist on rat spinal cord and nerve roots: light and electron microscopic observations after chronic intrathecal administration. Anesth Analg 1986; 65: 1303-1311. PMID: 2430489.


8. Coursin DB, Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care 2001; 7: 221-226. PMID: 11571417.


9. Elcicek K, Tekin M, Kati I. The Effects of intravenous dexmedetomidine on spinal hyperbaric ropivacaine anesthesia. J Anesth 2010; 24: 544-548. PMID: 20467879.


10. Fragen RJ, Fitzgerald PC. Effect of dexmedetomidine on the minimum alveolar concentration (MAC) of sevoflurane in adults age 55 to 70 years. J Clin Anesth 1999; 11: 466-470. PMID: 10526824.


11. Kaya FN, Yavascaoglu B, Turker G, Yildirim A, Gurbet A, Mogol EB, et al. Intravenous dexmedetomidine, but not midazolam, prolongs bupivacaine spinal anesthesia. Can J Anaesth 2010; 57: 39-45. PMID: 20039221.


12. Weinbroum AA, Szold O, Ogorek D, Flaishon R. The midazolam-induced paradox phenomenon is reversible by flumazenil. Epidemiology, patient characteristics and review of the literature. Eur J Anaesthesiol 2001; 18: 789-797. PMID: 11737177.


13. Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology 1992; 77: 1125-1133. PMID: 1361310.


14. Ustün Y, Gündüz M, Erdoğan O, Benlidayi ME. Dexmedetomidine versus midazolam in outpatient third molar surgery. J Oral Maxillofac Surg 2006; 64: 1353-1358. PMID: 16916668.


15. Cheung CW, Ying CL, Chiu WK, Wong GT, Ng KF, Irwin MG. A comparison of dexmedetomidine and midazolam for sedation in third molar surgery. Anaesthesia 2007; 62: 1132-1138. PMID: 17924894.


16. Erdurmus M, Aydin B, Usta B, Yagci R, Gozdemir M, Totan Y. Patient comfort and surgeon satisfaction during cataract surgery using topical anesthesia with or without dexmedetomidine sedation. Eur J Ophthalmol 2008; 18: 361-367. PMID: 18465717.


17. Ozaki M, Kurz A, Sessler DI, Lenhardt R, Schroeder M, Moayeri A, et al. Thermoregulatory thresholds during epidural and spinal anesthesia. Anesthesiology 1994; 81: 282-288. PMID: 8053576.


18. Usta B, Gozdemir M, Demircioglu RI, Muslu B, Sert H, Yaldiz A. Dexmedetomidine for the prevention of shivering during spinal anesthesia. Clinics (Sao Paulo) 2011; 66: 1187-1191. PMID: 21876972.




- TOOLS