Introduction
Patient-controlled analgesia (PCA) with opioids has been widely used for the control of post-operative pain. However, due to adverse effects such as nausea and vomiting, the adjuvant use of variable drugs has emerged [
1]. Among these, ketamine, a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist, has been known to improve postoperative opioid effectiveness [
2]. It has opioid sparing effect, inhibits windup and central sensitization, and reduces the development of chronic pain [
3-
5].
Low-dose ketamine is defined as a bolus dose of less than 2 mg/kg when given intramuscularly or less than 1 mg/kg when administered via the intravenous (IV) or epidural route. In continuous IV administration, low-dose ketamine is defined as a rate of ≤ 20 µg/kg/min [
6].
However, the effect of low-dose ketamine is controversial [
7-
10]. It is thought that the method and dose of ketamine administration, and differences in the intensity of postoperative pain, may affect the results [
11].
In this study, we investigated low-dose ketamine in terms of its opioid sparing effect in patients with IV PCA using fentanyl after lumbar spinal fusion surgery, which can cause severe postoperative pain.
Materials and Methods
Approval was obtained from the Institutional Review Board before study commencement. After receiving written informed consent, 60 healthy patients with an American Society of Anesthesiologists physical status classification of I-II, aged between 28 and 70 years old, and who were scheduled for elective major lumbar spinal surgery were enrolled in this randomized, placebo-controlled, double-blinded study. The type of surgery was posterior decompression and posterior lumbar interbody fusion with instrumentation.
The exclusion criteria comprised pregnancy, psychiatric problems, chronic alcoholism, drug abuse, inability to use PCA, and lack of communication ability.
Patients were randomly assigned to one of three groups: 1) K1 group, ketamine infusion of 1 µg/kg/min following bolus 0.5 mg/kg; 2) K2 group, ketamine infusion of 2 µg/kg/min following bolus 0.5 mg/kg of ketamine and 3) Control group, identical volume of normal saline infusion following bolus of normal saline before surgical incision. Continuous IV infusion of ketamine or saline following bolus dose started before skin incision intraoperatively, and continued until 48 h postoperatively via infusor (Autoinfusor®, Ace Medical Co, Seoul, Korea), which included the relevant study medication diluted with saline. All patients and the investigators collecting the postoperative data were blinded to the randomization.
A standardized anesthesia regimen was followed. All patients received glycopyrrolate 0.2 mg IM for premedication 30 min before surgery. Anesthesia was induced with propofol 2 mg/kg, remifentanil infusion 20 µg/min, and rocuronium 0.6 mg/kg. Anesthesia was maintained with desflurane, nitrous oxide (50%) and remifentanil infusion 5-20 µg/min. Approximate ten minutes before the end of surgery, a 50-100 µg bolus dose of fentanyl and 30 mg ketorolac were given intravenously. Ondansetron 8 mg was IV injected simultaneously and an additional 8 mg was mixed to PCA to prevent postoperative nausea and vomiting. After skin closure, desflurane and remifentanil were discontinued, and residual neuromuscular block was reversed with pyridostigmine and glycopyrrolate. The trachea was extubated when patients responded to verbal commands, and recovered spontaneous respiration. For postoperative pain control, patients were administered fentanyl using IV-PCA (bolus dose 15 µg of fentanyl, lockout interval of 5 min, no basal infusion). After surgery, patients were observed in the postanesthetic care unit for 1 h before transferal to the ward.
PCA fentanyl use was evaluated for 48 h after surgery. The pain was evaluated with a 100-mm visual analog scale (VAS) (0 = no pain; 100 = worst imaginable pain). Patients were asked to evaluate their maximal degree of pain. Pain scores were recorded at rest and with movement at 1, 6, 24, and 48 h after surgery. Pain with movement was defined as pain on rolling, sitting, or coughing.
Adverse events such as sedation, nausea, vomiting, headache and psychomimetic symptoms (vivid dreams or hallucination) were assessed. When moderate or severe nausea or vomiting was present, patients were administered metoclopramide 10 mg or ondansetron 4 mg. Patients were also asked to rate their overall satisfaction with the PCA experience on a five-point scale (very satisfied, satisfied, neutral, dissatisfied, very dissatisfied) [
12] at 48 h after operation.
The primary outcome measure of this study was the total amount of fentanyl consumption during the 48 h after operation, while the secondary outcome measures were the pain scores, adverse effects, and patient satisfaction.
Sample size was predetermined using a power analysis to achieve 90% chance (β = 0.1) with an assumed significance level of α = 0.05. From pilot study, the calculated minimum sample size was 17 patients in each group. A larger number of patients were included to allow for possible incomplete data collection or patient dropout. Data are presented as mean ± SD, or number of patients. The statistical analysis was performed using SPSS for Windows (version 14, SPSS Inc., Chicago, IL, USA). A one-way analysis of variance was used to compare the continuous variables among the groups. If a significant difference was noted, Bonferroni multiple comparison test was used to determine intergroup differences. Categorical variables were analyzed using the chi-square test or Fisher exact test, as appropriate. A P value of less than 0.05 was considered statistically significant.
Results
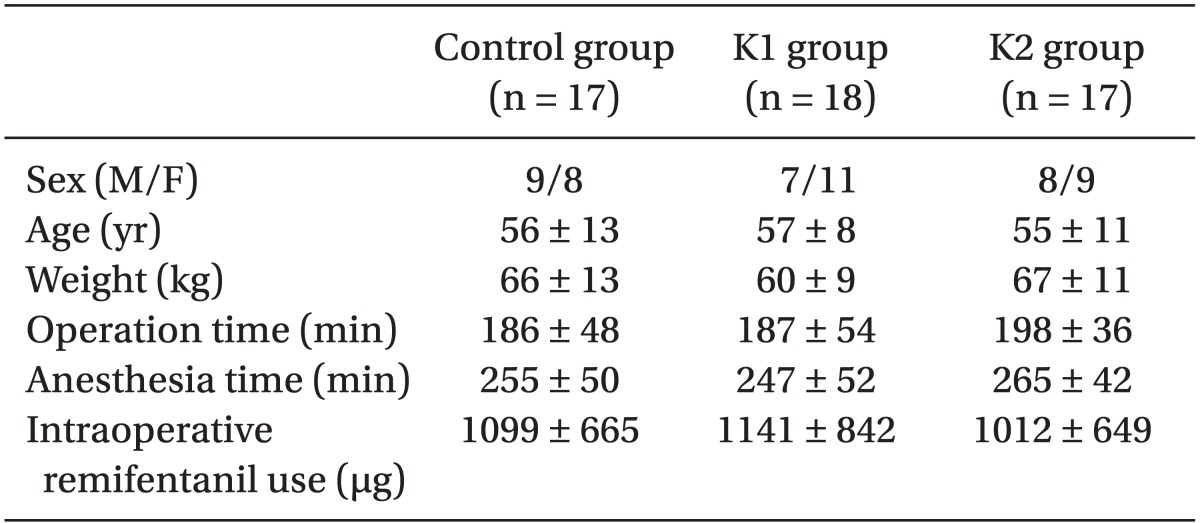
Of the 60 original patients, eight were lost from the study. Five patients stopped PCA use early because of severe postoperative nausea and vomiting (two patients in control, one patient in K1, two patients in K2), or because of PCA use error in three patients (one patient in control, one patient in K1, one patient in K2). There were no significant differences among the three groups with respect to demographic data, duration of operation and anesthesia, or the total amount of remifentanil used intraoperatively (
Table 1).
The total amount of fentanyl consumption was significantly lower in the K2 group (474 µg) compared to the control group (826 µg) and the K1 group (756 µg) during the 48 h after surgery (P < 0.05) (
Table 2).
VAS scores for pain at rest or with movement at 1, 6, 24 and 48 h postoperatively were similar among the three groups (
Table 2).
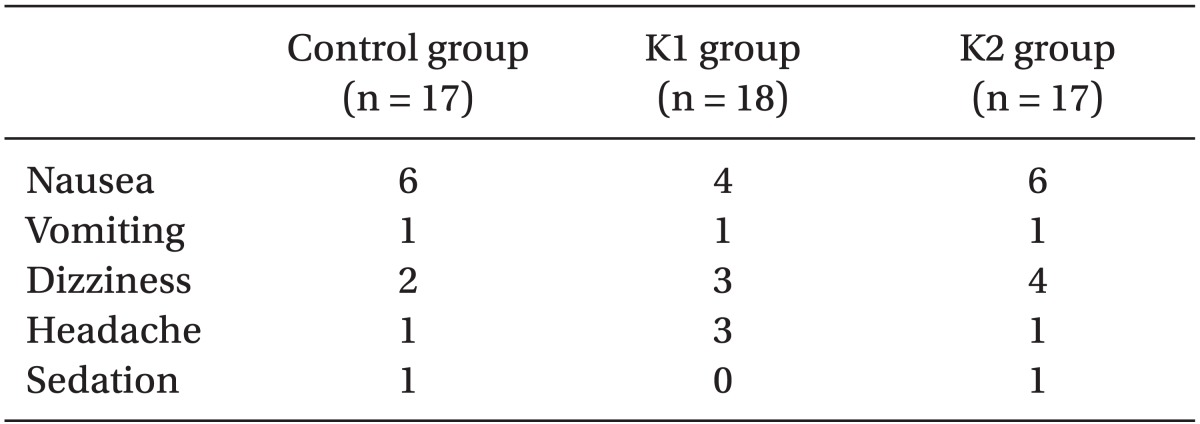
No patient experienced bad dreams or hallucinations. The incidence of adverse events such as nausea, vomiting, dizziness, headache, and sedation were comparable among the groups (
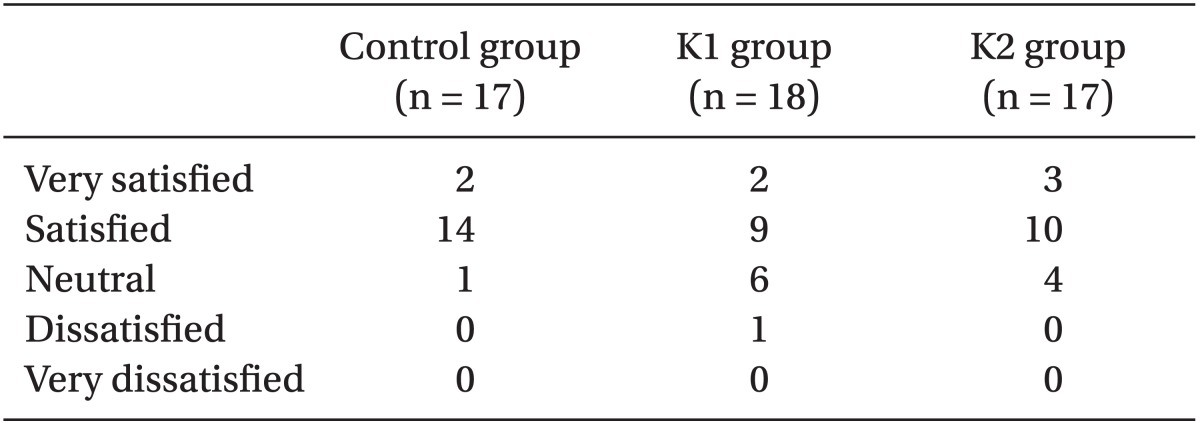
Table 3). The patient satisfaction rate was similar among the three groups (
Table 4).
Discussion
Ketamine has been used as a general anesthetic and analgesic for various pain conditions over the past several decades. Since Foster and Fagg reported the discovery of the NMDA receptor in 1987, ketamine has been used as a potential anti-hyperalgesic agent given its actions as a non-competitive NMDA-receptor antagonist. However, it remains a controversial drug due to undesirable adverse effects. It is clear that a distinction must be made between high-dose ketamine as an anesthetic agent and low-dose ketamine as an anti-hyperalgesic agent. There may even be a third dose range in which ketamine has no analgesic potency on its own but when used in combination with an opioid, yields an opioid sparing effect and superior pain relief than would occur for either drug alone [
6]. There is some indication that ketamine in a dose of 0.3 mg/kg does not interfere with µ-opiate receptors as, in this dose, its analgesic effects cannot be antagonized with the µ-opiate antagonist naloxone [
13]. Only interference with NMDA receptors may be involved in ketamine analgesia at this dose [
14], suggesting that 0.3 mg/kg ketamine may be "selective" for NMDA receptors [
15]. Furthermore, Tucker et al. [
16] reported that low steady doses of ketamine (serum ketamine 30-120 ng/ml) could be combined with µ opioid agonists to improve their analgesic effect without adverse effects in the clinical setting. In addition, for a ketamine infusion rate of 1-6 µg/kg/min in combination with a loading dose, there is evidence of anti-hyperalgesic, analgesic and opioid sparing effects [
17,
18]. We therefore investigated a loading dose of 0.5 mg/kg, followed by 1 or 2 µg/kg/min, as in these earlier studies.
In this study, the total amount of fentanyl used until 48 hours post-surgery was significantly lower in those patients who received ketamine infusion of 2 µg/kg/min following 0.5 mg/kg bolus dose compared to the those who received saline or ketamine infusion of 1 µg/kg/min following 0.5 mg/kg bolus dose. This result is consistent with other studies that used a similar dose [
19-
22]. In contrast with this result, however, Jaksch et al. [
23] reported that ketamine 2 µg/kg/min following 0.5 mg/kg bolus dose had no effect on postoperative pain reduction in patients after knee arthroscopic surgery, but this study differed from our own in that ketamine was infused for two hours post-surgery, whereas we infused for 48 hours.
Considering the short half-life of ketamine, the duration of infusion as well as the optimal dose is an important component of the opioid sparing effect. Zakine et al. [
24] compared ketamine infusion during only the intraoperative period with that for the perioperative period (intraoperative plus postoperative 48 h). They demonstrated that low dose ketamine improved postoperative analgesia with a significant decrease of morphine consumption when its administration was continued until 48 h postoperatively.
Another result from the current study is that there was no difference in fentanyl consumption between the K1 group (ketamine infusion of 1 µg/kg/min following 0.5 mg/kg bolus dose) and the control group. Yamauchi et al. [
25] reported that continuous low-dose ketamine improved analgesic effects after cervical spine surgery but not after lumbar surgery, and indicated that the ketamine dose required for the opioid sparing effect varies by the intensity of pain. Accordingly we investigated patients who underwent posterior lumbar interbody fusion with instrumentation, which has the capacity to cause similar severe pain. We also did not use basal infusion in PCA to prevent the masking effect of ketamine from pain reduction by continuous opioid infusion.
In this study, subjects did not report any psychomimetic effects such as bad dreams and hallucinations. There were no advantages observed in terms of pain VAS and a reduction in adverse effects despite lower opioid consumption in those patients who received a ketamine infusion of 2 µg/kg/min following 0.5 mg/kg bolus dose. As such, the opioid-sparing effect of ketamine in the clinical setting may be questionable, but may still be useful for patients who need a high dose of opioids or are unusually sensitive to opioids [
11].
The central limitation of this study is that we could not use higher doses of ketamine in light of potential psychomimetic effects. Other studies have used a similar dose without reporting adverse psychiatric effects, and Schmid et al. [
6] reported that the incidence of psychomimetic effects and cognitive impairment was negligible at dose less than 2.5 µg/kg/min IV and increased with higher doses. .The purpose of this study was to find the minimum effective dose of ketamine and as such further studies that assess what represents a clinically effective dose of ketamine may be needed.
In conclusion, low-dose ketamine of 2 µg/kg/min following bolus 0.5 mg/kg, but not not 1 µg/kg/min following bolus 0.5 mg/kg, significantly reduced the total amount of fentanyl consumption during 48 h after lumbar spinal fusion surgery without increasing the incidence of side effects.