|
 |
|
|
Abstract
Resection of large sacrococcygeal teratomas (SCTs) in premature neonates has been associated with significant perinatal mortality, making this a high risk procedure requiring careful anesthetic management. Most deaths during resection of SCTs are due to cardiac arrest caused by electrolyte imbalances, such as hyperkalemia, and massive bleeding during surgery. We describe two premature neonates who experienced cardiac arrest, one due to hyperkalemia and the other not due to hyperkalemia, during excision of large, prenatally diagnosed SCTs. We present here the considerations for anesthesia in premature neonates with huge SCTs.
Resection of large sacrococcygeal teratomas (SCTs) in premature neonates has the potential to be fatal and is associated with a high rate of perinatal mortality [1]. SCTs are the most common congenital neoplasms, occurring in 1 of 40,000 infants, with a female predominance of 95% [2]. Large SCTs are associated with significant perinatal morbidity and mortality (5-50%) [3]. Treatment consists of surgical removal of the sacral mass, which has hypervascularity derived from the middle sacral artery [1], and is usually performed immediately after birth [4]. Therefore resection of these tumors is a high risk procedure, requiring careful anesthetic management [5]. We describe here two premature neonates who experienced cardiac arrest during excision of a huge SCT.
A large SCT was detected by ultrasound in the fetus of a 27-year-old primagravida, with an unremarkable past medical history, at 25 weeks and 5 days of gestation. Because of preterm labor, the mother underwent an emergency cesarean section at 32 weeks and 2 days of gestation. The infant weighed 2.8 kg, with 1 and 5 minutes Apgar scores of 3 and 6, respectively. The patient was intubated and high frequency ventilation was required for poor respiration. Postnatal ultrasonography confirmed the presence of a highly vascular type 2 teratoma, 10 ├Ś 20 ├Ś 19 cm in size, extending to the pelvic and abdominal cavities, and containing solid and cystic components. There was also evidence of hydrops fetalis, including subcutaneous edema and severe abdominal ascites, and grade 4 hydronephrosis in both kidneys. Following a cystostomy, urine output was maintained at about 3-3.5 ml/kg/hr.
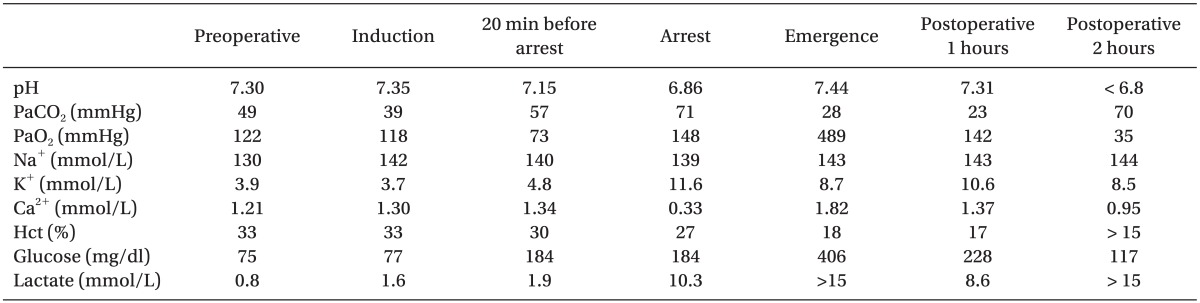
Preoperative laboratory results were as follows; hemoglobin 11 g/dl, hematocrit 33%, platelet 230 ├Ś 103/┬Ąl, prothrombin time 1.01 INR and activated partial thromboplastin time 38.4 seconds. Other electrolytes were within normal range (Table 1). Electrocardiogram (ECG) and chest x-ray were normal, but preoperative echocardiogram showed patent ductus arteriosus with left to right shunt, but revealed no congestive heart failure. On day 2 after birth, she was placed on a high frequency ventilation with improvements in her arterial blood gas analysis (ABGA). Thus, mechanical ventilator setting was changed to a synchronized intermittent mandatory ventilation mode. Surgery was scheduled on day 3 after birth. The patient arrived in the operating room without premedication. ECG, non-invasive blood pressure and oxygen saturation were monitored throughout. Anesthesia was induced with intravenous fentanyl (2 ┬Ąg/kg) and vecuronium (0.1 mg/kg) and maintained with 0.5 vol% isoflurane, a 30% oxygen/air mixture, and a continuous infusion of fentanyl (2 ┬Ąg/kg/hr). A 4-French central venous catheter (Arrow International, Inc, Pennsylvania, USA) was inserted into the left internal jugular vein to monitor central venous pressure and fluid replacement, and a 20-gauge peripheral intravenous line was inserted into the left antecubital area. A 24-gauge arterial catheter was inserted into the right brachial artery for continuous monitoring of arterial blood pressure and frequent ABGA. Since an intra-abdominal tumor would make ventilation difficult, surgeon performed an abdominal incision and decompression. Ventilator was set to a pressure control mode with peak inspiratory pressure of 25 mmHg, tidal volume of 30 ml and respiratory rate of 30 breaths/min. The patient was placed in the prone position for excision of sacrococcygeal mass. Temperature maintained at 35.5-37.0Ōäā using a force warming device and an intravenous fluid warmer. Bleeding continued during surgery, with hemoglobin concentration declining to 6.1 g/dl and hematocrit to 18%, requiring aggressive transfusion with packed red blood cells (pRBCs). The pRBCs, fresh-frozen plasma (FFP) and platelet concentrates (PC) were transfused throughout the procedure. About 2 hours later, adequate ventilation was maintained. An arterial partial pressure of oxygen was 118 mmHg and oxygen saturation was 100% on 30% mixed with medical air. Following the dissection of the posterior aspect of tumor, which was 2 hours and 40 minutes after induction, the patient was placed in the supine position for manipulation of the abdominal mass. During resection of the abdominal mass, respiratory ventilation was difficult because the tumor pressed onto the chest. Peak inspiratory pressure was increased to 35 mmHg, and tidal volume was reduced to 15 ml. Therefore, we frequently had to ventilate manually. Also, ABGA showed hypoxemia, hypercarbia and respiratory acidosis (Table 1). After repeating this process for about 20 minutes, there was a sudden hypotension and ventricular fibrillation on ECG followed by extreme bradycardia and asystole. At the time of the cardiac arrest, body temperature was 37.0Ōäā. Resuscitation was successfully performed with external cardiac massage, repeated injections of epinephrine 10 ┬Ąg, and continuous infusion of dopamine (5 ┬Ąg/kg/min). During resuscitation, ABGA showed severe hyperkalemia with a value of 11.6 mmol/L (Table 1), which was managed by repeated injections of calcium gluconate 30 mg and sodium bicarbonate 3 mEqL and continuous infusion with regular insulin (0.4 u/hr). The total duration of cardiac arrest was 30 minutes. Mean arterial pressure was maintained at 40-50 mmHg during resuscitation. Following resuscitation, the surgeons decided to proceed with the operation. The patient's potassium level decreased to 8.7 mmol/L, but remained high (Table 1). The total operation time was 5 hours, during which the patient received a total of 230 ml normal saline, 580 ml pRBCs, 240 ml FFP, and 40 ml PC. Urine output gradually decreased during surgery, with a total urine output of 25 ml.
The patient was transferred to the neonatal intensive care unit (NICU) in relatively stable condition, except for poor urination. In the NICU, however, intra-abdominal bleeding continued because of disseminated intravascular coagulation, and the patient required pRBCs, FFP and PC. Despite regular infusions of insulin, she showed persistent hyperkalemia (Table 1) accompanied by oliguria, suggesting a congenital urinary tract anomaly such as urethral malformation or obstruction. The patient's heart rate and blood pressure gradually decreased, leading to cardiovascular collapse. Despite prolonged cardiac massage for about 2 hours, the patient died. A pathological examination confirmed the diagnosis of SCT, with a malignant mixed germ cell tumor.
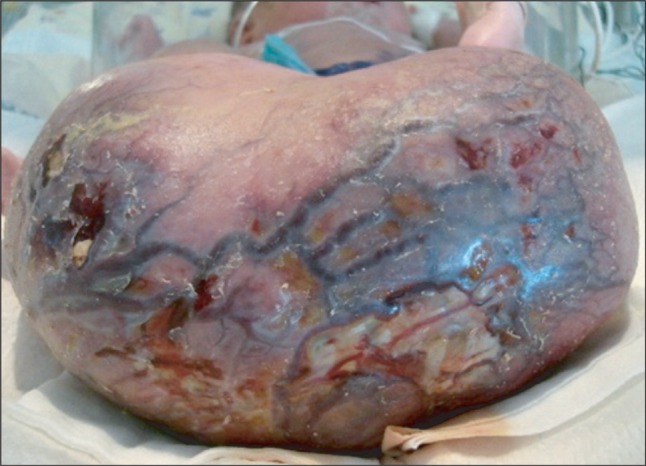
A large mass, measuring about 15 ├Ś 13 ├Ś 10 cm, was detected by ultrasound in the fetus of a 30-year-old primagravida at 26 weeks and 3 days of gestation. Radiofrequency ablation was performed in utero at 31 weeks and 1 day of gestation, but the size of the tumor was not markedly reduced. Prenatal ultrasound showed no evidence of hydrops fetalis or other anomalies. Because of the premature rupture of membranes, the mother underwent an emergency cesarean section at 35 weeks and 5 days of gestation. The baby weighed 3.7 kg, with 1 and 5 minutes Apgar scores of 6 and 9, respectively. Ulceration and bleeding were seen on the surface of the SCT (Fig. 1), but hyperkalemia did not developed.
Preoperative laboratory results, ECG, chest x-ray and echocardiogram were normal. Surgery was scheduled on day 3 after birth. In the operating room, the patient was monitored by ECG, non-invasive blood pressure and oxygen saturation. Anesthesia was induced with 2 ┬Ąg/kg fentanyl and muscle relaxation was achieved with 0.1 mg/kg vecuronium. General anesthesia was maintained with sevoflurane at 0.5 vol% and a continuous infusion of fentanyl (2 ┬Ąg/kg/hr). A 5-French central venous catheter (Arrow International, Inc, Pennsylvania, USA) was inserted into the right internal jugular vein to monitor central venous pressure and administer fluid, and a 22 gauge peripheral intravenous line was inserted into the right antecubital area. A 24 gauge arterial catheter was inserted into the right brachial artery for continuous arterial blood pressure monitoring and frequent ABGA. The patient was then placed in the prone position for the procedure, because the mass was located in the sacrococcygeal area. After positioning, ABGA showed normal values (Table 2), which confirmed adequate ventilation. Temperature was maintained at 35.5-36.5Ōäā using a force warming device and an intravenous fluid warmer. During operation, bleeding continued, with hemoglobin levels dropping to 5.4 g/dl and hematocrit to 16%, requiring aggressive transfusion of pRBCs. The pRBCs, FFP, PC and cryoprecipitate were transfused throughout the procedure. Two hours after skin incision, the patient's heart rate dropped from 140 bpm to below 80 bpm and blood pressure suddenly dropped from 58/37 mmHg to 28/15 mmHg. At the time of the circulatory collapse with marked bradycardia, body temperature was 36.1Ōäā. Resuscitation was successfully performed with external cardiac massage and repeated injections of epinephrine 10 ┬Ąg and atropine 20 ┬Ąg. During resuscitation, ABGA showed severe metabolic and respiratory acidosis and hypocalcemia, but potassium concentration was within normal limits (Table 2). The duration of cardiac massage was about 1 min, after which the surgeons decided to proceed with the surgery. The total duration of the operation was 5 hours, during which the patient received a total of 100 ml normal saline, 40 ml lactated Ringer's solution, 660 ml pRBCs, 120 ml FFP, 240 ml PC and 40 ml cryoprecipitate. Total urine output was 34 ml. The patient was transferred to the NICU in a stable condition. She was extubated on postoperative day 6 and discharged home in good condition on postoperative day 17. A pathological examination confirmed the diagnosis of SCT, consisting of an immature teratoma without malignant cells.
A large vascular SCT carries a high risk of prenatal complications, including high-output cardiac failure and fetal hydrops caused by arteriovenous shunting through the tumor. Therefore preterm neonates with large SCTs are at very high risk of death [1]. Although the mortality rate for newborns with SCT is at most 5%, the mortality rate for fetal SCT exceeds 50%, and fetal SCT associated with non-immune hydrops is uniformly fatal [3]. Predictors of poor outcome include diagnosis before 20 weeks gestation, delivery before 30 weeks, low birth weight, Apgar score less than 7, malignant histotypes, polyhydramnios, placentomegaly and development of hydrops [2].
Treatment for SCTs consists of surgical removal of the sacral mass [4]. Surgical resection should be performed immediately after birth as coagulopathy appears to worsen with time [6]. The etiology of the coagulopathy associated with SCTs is unclear, but appears to be multifactorial, including prenatal diagnosis, polyhydroamniosis, large sized (> 10 cm) tumor, prematurity and hydrops fetalis [7]. Surgical removal of a SCT is a high risk procedure and requires careful anesthetic management [4].
During the operation, respiratory ventilation is difficult because of the tumor pressing onto the chest, and that may be worsen by prone position. Abraham et al. [2] has been reported that the manual ventilation and lifting the tumor by surgeon can help with the ventilation. And it is very difficult to maintain body temperature because of the large surface area of the SCT compared to the patient. Hypothermia could worsen coagulopathy, and prolong the effect of the anesthetics [2]. In order to maintain body temperature, thermoneutral incubator, force warming device, and intravenous fluid warmer should be used.
Most clinical reviews of SCT have reported that the cause of death is cardiac arrest due to electrolyte imbalances, especially hyperkalemia [8]. Hyperkalemia, which may have been associated with manipulation of the tumor during resection, especially manipulating the tumor for intra-abdominal resection, and massive transfusion of pRBCs containing high levels of potassium [4]. In addition, metabolic and respiratory acidosis, hypocalcemia, hypothermia and oliguria may worsen hyperkalemia [9]. Hypoxemia and hypovolemia also contributed to cardiac arrest [4].
Concerning case 1, as respiratory ventilation was expected to be difficult, the surgeon performed abdominal decompression immediately after induction. Hence, adequate ventilation was maintained during resection of sacral mass. However, during manipulation of intra-abdominal mass, respiratory ventilation was difficult because of the tumor pressing onto the chest. Therefore, we frequently had to ventilate manually and had the tumor lifted by surgeon, but acidosis had continued to get worse. In addition, severe hyperkalemia, which may have been released from necrotic foci on the tumor, had caused cardiac arrest. In particular, severe acidosis caused by difficult ventilation and massive transfusion of pRBCs containing high levels of potassium would have worsen hyperkalemia. While in case 2, circulatory collapse without hyperkalemia suddenly occurred, which is probably thought to be due to persistent bleeding.
However, even though both of our patients showed hemodynamic instability, the occurrence of massive hemorrhage was not recognized. Moreover, the amount of bleeding was not correlated with emergency laboratory results. Mortality secondary to blood loss, either into the tumor or during surgery, has also been reported in neonates with SCT. For example, post-surgical deaths due to bleeding were reported in 9 of 247 patients [10] and in 4 of 41 neonates with SCT [11]. Especially, the occurrence of intra-tumoral hemorrhage may mask bleeding, so frequent laboratory tests should be prepared for massive bleeding.
SCTs are now often prenatally diagnosed by ultrasound. Treatments in utero include cyst aspiration, open fetal surgery, radiofrequency ablation, and thermocoagulation, with variable success, but there have been no large case series [12]. Preoperative radiologic embolization can effectively reduce blood loss during surgery and enable faster and safer resection of the tumor [1]. Therefore, preoperative embolization should be considered essential in preventing complications.
Our findings indicate the importance of careful anesthetic management during resection of SCTs, since this operation takes high risk, especially in neonates. In particular, hyperkalemia, associated with tumor lysis syndrome and massive transfusion, can lead to cardiac arrest [8]. A surgical manipulation of very advanced solid tumors can increase the probability of tumor lysis [4]. Consequently, if there are multiple necrotic foci on the tumor, surgeon should carefully manipulate the tumor to minimize tumor lysis. In addition, during resection of SCTs, unrecognized intratumoral bleeding often occurs and requires massive transfusion. Therefore, we should be vigilant monitoring and frequent laboratory tests, especially potassium and hemoglobin, before and during surgery are necessary.
In conclusion, during resection of huge SCTs, which contain multiple necrotic foci, in neonates, anesthesiologist should be aware of possibility of difficult ventilation and hyperkalemia. Also, preoperative embolization should be performed for the prevention of complications.
References
1. Lahdes-Vasama TT, Korhonen PH, Seppanen JM, Tammela OK, Iber T. Preoperative embolization of giant sacrococcygeal teratoma in a premature newborn. J Pediatr Surg 2011; 46: e5-e8. PMID: 21238631.

2. Abraham E, Parray T, Ghafoor A. Complications with massive sacrococcygeal tumor resection on a premature neonate. J Anesth 2010; 24: 951-954. PMID: 21057822.


3. Robertson FM, Crombleholme TM, Frantz ID 3rd, Shephard BA, Bianchi DW, D'Alton ME. Devascularization and staged resection of giant sacrococcygeal teratoma in the premature infant. J Pediatr Surg 1995; 30: 309-311. PMID: 7738756.


4. Reinoso-Barbero F, Sepulveda I, Perez-Ferrer A, De Andres A. Cardiac arrest secondary to hyperkalemia during surgery for a neonatal giant sacrococcygeal teratoma. Paediatr Anaesth 2009; 19: 712-714. PMID: 19638127.


5. Robinson S, Laussen PC, Brown TC, Woodward AA. Anaesthesia for sacrococcygeal teratoma--a case report and a review of 32 cases. Anaesth Intensive Care 1992; 20: 354-358. PMID: 1524178.


6. Girisch M, Rauch R, Carbon R, Habash T, Hofbeck M. Refractory bleeding following major surgery of a giant sacrococcygeal teratoma in a premature infant: successful use of recombinant factor VIIa. Eur J Pediatr 2004; 163: 118-119. PMID: 14716558.


7. Murphy JJ, Blair GK, Fraser GC. Coagulopathy associated with large sacrococcygeal teratomas. J Pediatr Surg 1992; 27: 1308-1310. PMID: 1403511.


8. Jona JZ. Progressive tumor necrosis and lethal hyperkalemia in a neonate with sacrococcygeal teratoma (SCT). J Perinatol 1999; 19: 538-540. PMID: 10685309.



9. Smith HM, Farrow SJ, Ackerman JD, Stubbs JR, Sprung J. Cardiac arrests associated with hyperkalemia during red blood cell transfusion: a case series. Anesth Analg 2008; 106: 1062-1069. PMID: 18349174.


10. Altman RP, Randolph JG, Lilly JR. Sacrococcygeal teratoma: American Academy of Pediatrics Surgical Section Survey-1973. J Pediatr Surg 1974; 9: 389-398. PMID: 4843993.


11. Grosfeld JL, Ballantine TV, Lowe D, Baehner RL. Benign and malignant teratomas in children: analysis of 85 patients. Surgery 1976; 80: 297-305. PMID: 960000.

12. Hedrick HL, Flake AW, Crombleholme TM, Howell LJ, Johnson MP, Wilson RD, et al. Sacrococcygeal teratoma: prenatal assessment, fetal intervention, and outcome. J Pediatr Surg 2004; 39: 430-438. PMID: 15017565.


- TOOLS