|
 |
|
|
Abstract
The process of micturition is related to activation of the cardiovascular autonomic nervous system. Hypotension with bradycardia often occurs during or immediately after micturition. We experienced a case of sudden severe hypotension and bradycardia following urethral catheterization in a patient who underwent an urethral dilatation and transurethral resection of bladder tumor while under general anesthesia. The patient was treated with inotropics and intravenous fluids, and he recovered without any complications. The characteristics of this case are similar to the physiologic changes that occur in micturition syncope. Therefore, it is presumed that the autonomic reflex that was triggered by the urethral catheterization caused the hypotension and bradycardia.
Micturition, which is a complex mechanism that depends on neural and voluntary actions, is facilitated or inhibited by various reflexive actions. While the micturition reflex that occurs by bladder distention contracts the bladder and relaxes the urethral sphincter to start the urination, the urethrovesical reflex that occurs by urethral distention on urination contracts the bladder to maintain and complete the voiding act [1]. The process of micturition is related to activation of the cardiovascular autonomic nervous system. That is, cardiovascular autonomic activity in which the blood pressure (BP) and the heart rate (HR) are reduced due to a sudden pressure reduction of the expanded bladder correlates with urinary autonomic activity, in which the sympathetic supply is inhibited and the parasympathetic supply is excited [2]. We report on a patient who was treated with urethral dilatation and transurethral resection of bladder tumor (TURBT) while under general anesthesia and who experienced severe hypotension after insertion of the urinary catheter.
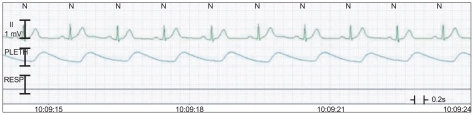
A 65-year-old male patient was scheduled for urethral dilatation and TURBT due to dysuria. According to his medical history, he had been taking oral hypoglycemic agents because of diabetes that had been diagnosed 2 months before, and his blood glucose levels were well controlled. He had undergone a left nephroureterectomy for a left proximal ureter tumor 3 years before. In addition, he had undergone TURBT for a bladder tumor, visual internal urethrotomy, and a prostate biopsy for multiple urethral strictures 2 years before. At that time, he was diagnosed with benign prostatic hypertrophy (BPH). During those procedures, he had no problems with general anesthesia. Preoperative laboratory findings, electrocardiogram (ECG) results, echocardiography results, and pulmonary function tests were all within normal limits (Fig. 1). He was not premedicated and was transferred to the operating room where ECG (lead II), noninvasive blood pressure, and pulse oximetry monitors were applied. Thiopental sodium (300 mg) and Atracurium (30 mg) were injected intravenously for the induction of anesthesia, and the trachea was intubated. The anesthesia was maintained with O2 (2 L/min), N2O (2 L/min), sevoflurane (1-2 vol%), and remifentanil (0.05 mcg/kg/min).
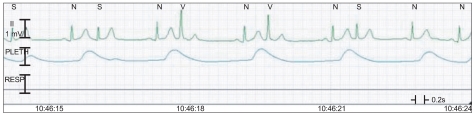
The surgery was started after 15 min of anesthesia induction, and the vital signs that were monitored during the surgery were a BP of 120-140/60-80 mmHg, a HR of 70-80 beats/min, oxygen saturation of 100%. There were no abnormal findings on the ECG. The surgery was finished in 30 min, and a 3-way foley catheter was inserted for the prevention of urethral stenosis and acute urinary retention from thrombi in the bladder and for bladder irrigation. More than 400 ml of diluted hematuria that was mixed with bladder irrigation fluid, urine, and residual blood in bladder drained in an instant. About 10 sec later, his BP and HR abruptly decreased to 90/45 mmHg and 50 beats/min. Ephedrine (5 mg) and glycopyrrolate (0.2 mg) were injected immediately. Soon after, the ECG showed an atrial premature complex (APC) (Fig. 2). After the intravenous administration of 2% lidocaine (80 mg), the APC disappeared. However, after 10 min, BP decreased to 70/35 mmHg, and HR increased to 100 beats/min (Fig. 3). We immediately changed the patient's position to a head-down position and intravenously administered ephedrine (10 mg) and phenylephrine (200 ┬Ąg) in 2 divided doses. Nevertheless, his BP did not increase. Therefore, epinephrine (100 ┬Ąg) was intravenously injected 2 times, and a 16-gauge catheter was placed in the right external jugular vein for the rapid administration of colloid solution. In addition, a left radial artery catheter was placed for continuous BP monitoring. However, the BP decreased to 60/30 mmHg, and a ST segment elevation appeared on ECG. Dopamine (5 ┬Ąg/kg/min) and isosorbide dinitrate (0.5 ┬Ąg/kg/min) were infused continuously with careful monitoring (Fig. 4). Arterial blood gas analysis results at that time were pH, 7.248; PaCO2, 38.2 mmHg; PaO2, 84.1 mmHg; HCO3-, 16.3 mEq/L; BE, -11 mEq/L; and SaO2, 94.6%, which showed metabolic acidosis, Hgb, 15.8 g/dl; Hct, 46%; and normal electrolytes. Soon after, the ST segment gradually became normal, but the BP did not. Thus, additional epinephrine (30 ┬Ąg) was injected while vital signs were monitored. Thirty min after the hypotension and bradycardia occurred, tachycardia was still apparent, but BP improved. The patient was transferred to the intensive care unit (ICU) for careful monitoring after a discussion with his attending surgeon. During the 15 min of waiting for transfer, the patient's BP and HR were maintained at 100/60 mmHg and 108-115 beats/min, respectively. Arterial blood gas analysis results at that time were pH, 7.248; PaCO2, 44.6 mmHg; PaO2, 105.8 mmHg; HCO3-, 18.8 mEq/L; BE, -8.4 mEq/L; SaO2, 96.9%; Hgb, 14.7 g/dl; Hct, 43%; and normal electrolytes. Blood loss volume during the operation was 200 ml, infused crystalloid fluid was 1,300 ml, infused colloid solution was 400 ml, and urine volume was 430 ml. We started to wake the patient slowly and extubated after confirming that the patient had recovered his breath and consciousness. The patients was then transferred to the ICU.
In the ICU, the ST segment on ECG improved completely. The isosorbide dinitrate infusion was stopped, but BP was not well maintained around 80/50 mmHg. Thus, dopamine was increased to 7-10 ┬Ąg/kg/min, and BP recovered normally. Ten h after the surgery was finished, BP and HR were stabilized at 110/60 mmHg and 60-70 beats/min, respectively. The infusion dose of dopamine was gradually decreased, and 16 h after surgery, the infusion was stopped. Blood cell counts were maintained at normal levels with Hgb, 13.3 g/dl and Hct, 43%. The results of the myocardial enzyme test conducted 4 h after the hypotension had occurred showed normal CPK and CK-MB at 93 U/L and 2.34 U/L, respectively, and Troponin T was slightly increased from the upper limit of normal at 0.321 ng/ml. Seven h after hypotension occurred, CPK and CK-MB were also normal, and Troponin T decreased slightly to 0.192 ng/ml. Sixteen h after hypotension occurred, Troponin T decreased to the normal range. The patient was transferred to a general ward after recovery and was discharged 15 days after surgery with normal results on follow-up tests of ECG, myocardial enzymes, and blood tests and without any symptoms or complications.
The main causes of hypotension during anesthesia are drugs, such as intravenous induction agents or inhalational agents (26%), regional anesthesia (14%), hypovolemia due to bleeding and dehydration (9%), and so on [3]. Fifty-one percent of incidents of bradycardia that occurred during anesthesia was accompanied by hypotension, and common causes of bradycardia that is accompanied by hypotension during anesthesia are drugs (30%), regional anesthesia (16%), autonomic reflex (16%), and others, such as airway and cardiopulmonary problems, hypovolemia, allergies, and other unknown causes. Vagal reflex could be induced by operational causes, such as vagal stimulation in the abdominal cavity or pelvis and oculocardiac reflex, or anesthetic causes, such as laryngoscopy and manipulation of wires or catheters in central venous cannulation [4]. In our case, the patient was under general anesthesia with intubation and had no obvious cardiopulmonary abnormalities on preoperative tests and no signs of dehydration with the continuous administration of fluids for the supplementation of intravenous volume. In addition, due to the results of the blood tests that were performed after the hypotension occurred, it seemed that excessive blood loss, which can lead to hypovolemia, did not happen. Moreover, there was no additional drug administration except for the insertion of the 3-way foley catheter before the hypotension and bradycardia occurred, and no doubtful allergic skin reactions were seen. Therefore, we suggest that the occurrence of an autonomic reflex that was induced by insertion of the foley catheter was the most probable cause.
Micturition syncope, which occurs in arousal state patients, causes physiological changes that are similar to this case. Syncope is a transient loss of consciousness that results from an insufficient supply of oxygen to the brain when cerebral blood flow falls to below about half of the normal value [5]. Micturition syncope mainly occurs during or after voiding when the patient gets up from sleep at night or in the morning. It accounts for 8.39% of all syncope and usually occurs in healthy men, but it is rare. These episodes usually present as bradycardia, hypotension, and loss of consciousness, and the event usually lasts less than 30 min with full recovery. Circulatory disturbances, such as standing position, alcohol consumption, airway infection, fatigue, warmth, hypnic deprivation, and the valsalva maneuver are known trigger factors of micturition syncope [2,6-8]. Sumiyoshi et al. [9] studied age-dependent clinical characteristics of micturition syncope. Micturition syncope in the younger group tended to occur in the evening or nighttime before midnight, whereas micturition syncope in the older group over 55 years tended to occur after midnight or early in the morning. Alcohol intake was an important precipitating factor in the younger group. Micturition syncope can be an isolated symptom in healthy subjects, but it is frequently associated with other neurological causes, such as autonomic failure of primary (multiple system atrophy; MSA) or secondary (such as diabetes) etiology. In addition, it was reported that intermittent catheterization caused micturition syncope in a quadriplegic patient due to cervical spinal cord injury that was accompanied with autonomic dysreflexia [2,10].
The mechanism of micturition syncope is not fully understood, but there have been some suggestions from animal experiments and patient studies. First, the action of standing up from lying down a long time at night when vagal tone is increased and the decrease of baroreceptor function during sleep could explain why micturition syncope is related to sleep. An excessively distended bladder delivers strong afferent stimulation to the central nervous system, and sudden decompression of a distended bladder by urination results in a diminution of sympathetic tone. In addition, strong parasympathetic activation by vagal discharge that is produced by the act of voiding can further decrease an already low BP. At this point, the relief of bladder distension could cause vasodilatation because of reduced stimulation to the bladder stretch receptors. Moreover, urination of a patient with bladder neck stenosis or obstruction may trigger the valsalva maneuver, which increases thoracic and intraabdominal pressure, and subsequently diminishes venous return. Therefore, it is presumed that micturition syncope can cause hypotension and bradycardia not by any single entity, but by a complex of factors [5-8,11].
In the study of patients who experienced micturition syncope by Sakakibara et al. [12], they showed that a bladder filling with water in the supine lithotomy position caused minimal increases of BP and HR, changing to a sitting position with a distended bladder caused mild orthostatic hypotension, and bladder evacuation caused decreases in BP and HR. This is similar to vasovagal syncope and suggests that afferent input from urination may be important. In a comparative study of patients with MSA and normal controls by Uchiyama et al. [2], they found that, compared with normal controls, patients with MSA had a lower baseline BP, smaller increases in BP and HR during bladder filling, and a marked decrease in BP for a longer duration when urinating. Micturition syncope is common in patients with MSA, and this is presumed to be a result of abdominal straining due to dysuria in MSA patients and general autonomic insufficiency that is estimated to be abnormal in a dominant parasympathetic state. In addition, Ishikawa et al. [13] found that, in a study of patients who did not have neurological and urological disorders, a foley catheter was inserted into the bladder, and the bladder was filled with saline. When the infused saline was drained suddenly, decreases in mean BP and HR was minimal under light general anesthesia without inhibiting autonomic response in the supine position. However, they observed unstable cardiovascular changes in several subjects, and this was explained by variable autonomic responsiveness and the distension level of the bladder between individuals.
The patient in this case had never experienced micturition syncope, had no neurological disorders, and had a low possibility of autonomic neuropathy because he was diagnosed with diabetes only 2 months before. In addition, urination through catheterization under anesthesia makes abdominal straining impossible. Morover, it is different from the features of micturition syncope in a general arousal state because of the supine position, and no cases that are similar to this patient have been reported. However, when aggregating all of the studies mentioned above, it is presumed that, if an autonomic response was not fully inhibited under this patient's anesthetic depth, there was a possibility of an induction of a parasympathetic autonomic reflex due to a sudden decrease of bladder volume by the drainage of a large amount of urine through the foley catheter while the patient was in a supine position. Moreover, it is presumed that the anesthetic effect led to vasodilatation and attenuation of the physiological compensatory mechanism and induced more serious hypotension in association with the strong autonomic reflex. When considering the patient's surgical history due to his bladder tumor, his diagnostic history of multiple urethral stenosis and BPH, and his dysuria as the chief complaint, a high possibility of urinary retention in the bladder could act as a triggering factor.
After transfer to the ICU, the patient had a consultation with cardiology, and follow-up ECG and angiography were contemplated in order to consider the possibility of a coronary artery problem in case the dopamine tapering was difficult due to continuous hypotension. However, the patient's ECG recovered to normal, and vital signs were stable after stopping dopamine. Cardiac enzymes were all normal except that Troponin T was slightly increased from the upper limit of normal after 4 h of hypotension occurrence and then continuously decreased. Thus, cardiac complications by intraoperative ischemia seemed not to have occurred, and the patient was discharged without additional tests. However, there is a question of whether the patient showed continuous hypotension after arousal, which needed inotropics, even though the recovery of the autonomic reflex was slow due to anesthetic effects, while most micturition syncope recovers within 30 min. Therefore, when considering all factors, such as that the ST segment was increased transiently and recovered to normal soon after, the hypotension continued for about 10 h after surgery, and everything was recovered to normal without cardiac complications, the authors presume that hypotension by autonomic reflex induced temporary myocardial ischemia, which induced a reversible myocardial depression that continued for hours and then fully recovered.
In conclusion, this case shows that the drainage of a large amount of urine by urethral catheterization from a distended bladder while the patient is in a supine position and under anesthesia is similar to micturition syncope, but it can cause more serious hypotension and bradycardia. The autonomic reflex during urination may be the main cause, which is similar to the mechanism of micturition syncope, and it is suggested that the effects of residual anesthetics had a role in decreasing the BP even more. In addition, temporary myocardial ischemia due to hypotension induced myocardial depression, and hypotension continued as a result. Therefore, we should consider that the sudden drainage of urinary retention by urethral catheterization while the patient is under anesthesia can induce hypotension and bradycardia, and vital signs should be monitored carefully.
References
1. Shafik A, el-Sibai O, Ahmed I. Effect of urethral dilation on vesical motor activity: identification of the urethrovesical reflex and its role in voiding. J Urol 2003; 169: 1017-1019. PMID: 12576835.


2. Uchiyama T, Sakakibara R, Asahina M, Yamanishi T, Hattori T. Post-micturitional hypotension in patients with multiple system atrophy. J Neurol Neurosurg Psychiatry 2005; 76: 186-190. PMID: 15654029.



3. Morris RW, Watterson LM, Westhorpe RN, Webb RK. Crisis management during anaesthesia: hypotension. Qual Saf Health Care 2005; 14: e11PMID: 15933284.



4. Watterson LM, Morris RW, Westhorpe RN, Williamson JA. Crisis management during anaesthesia: bradycardia. Qual Saf Health Care 2005; 14: e9PMID: 15933306.



5. Hainsworth R. Pathophysiology of syncope. Clin Auton Res 2004; 14(Suppl 1): 18-24. PMID: 15480926.


6. Schiavone A, Biasi MT, Buonomo C, Nozzoli C, Roca ME, Sambati R, et al. Micturition syncopes. Funct Neurol 1991; 6: 305-308. PMID: 1743547.

8. Kao YJ, Racz GB. Loss of consciousness after emergence from anaesthesia. A case of suspected micturition syncope. Anaesthesia 1990; 45: 738-740. PMID: 2240534.


9. Sumiyoshi M, Abe H, Kohno R, Sekita G, Tokano T, Nakazato Y, et al. Age-dependent clinical characteristics of micturition syncope. Circ J 2009; 73: 1651-1654. PMID: 19597300.


10. Previnaire JG, Soler JM. Micturition syncope following intermittent catheterisation in a tetraplegic patient. Spinal Cord 2006; 44: 695-696. PMID: 16462821.



11. Mary DA. The urinary bladder and cardiovascular reflexes. Int J Cardiol 1989; 23: 11-17. PMID: 2654028.


12. Sakakibara R, Hattori T, Kita K, Yamanishi T, Yasuda K. Urodynamic and cardiovascular measurements in patients with micturition syncope. Clin Auton Res 1997; 7: 219-221. PMID: 9370067.


13. Ishikawa T, Sato J, Nishino T. Acute changes in bladder volume produce minimal cardio-respiratory responses in lightly anesthetised humans. Can J Anaesth 2000; 47: 786-791. PMID: 10958096.


- TOOLS
-
METRICS

-
- 0 Crossref
- 1 Scopus
- 3,116 View
- 36 Download