 |
 |
|
|
Abstract
Background
Reactive oxygen species (ROS) such as superoxide radicals, hydrogen peroxide, nitric oxide, and nitroperoxide, cause oxidative stress which interferes with normal cell functioning, resulting in cell damage. It is reported to be associated with chronic pain, especially neuropathic pain, and inflammatory pain. ROS is also closely related to central sensitization. Therefore, this study was designed to explore the effects of Phenyl N-tert-butylnitrone (PBN), an ROS scavenger, in acute, continuous, and increasing pain caused by central sensitization.
Methods
Male Sprague-Dawley rats were divided into 2 groups, an intraperitoneal group (IP) and an intrathecal group (IT), and once again divided into an experimental group and a control group. The experimental group was injected with Phenyl N-tert-butylnitrone (PBN), a free radical scavenger, either intraperitoneally or intrathecally. After inducing pain by injecting formalin into the hind paw, pain behaviors were measured. Lumbar enlargement immmunohistochemistry was performed to assess nitrotyrosine, an oxidative stress marker, to identify the degree of protein nitration.
Results
Both experimental groups of IP and IT showed statistically significant decreases in the number of flinches compared to the control group in phase 1 and 2. Immunohistochemical evaluation in the control group revealed an increase in nitrated proteins in the gray matter of the lumbar spinal cord, but a significant decrease in nitrated proteins in the gray matter of lumbar spinal cord of the experimental group.
Pain is a common reason for deteriorating life quality and productivity [1]. Currently, anti-inflammatory and narcotic analgesics are primarily used, but their side-effects are unsatisfactory. Response to narcotics is poor, especially to neuropathic pain, and the need for higher dosages can lead to complications [2]. Among studies regarding the pathophysiology of neuropathic pain, some claim that the mechanism is due to an overproduction of reactive oxygen species (ROS). Therefore, using an ROS antagonist would be effective, bringing anticipation for a new analgesic [3,4].
Oxidative stress due to ROS, such as superoxide radicals, hydrogen peroxide, nitric oxide, and nitroperoxide, interferes with normal cell function that results in cell damage. According to recent studies, ROS is reported to be associated with chronic pain, especially neuropathic pain, and inflammatory pain. In addition, ROS is closely related to central sensitization [3,4]. The N-methyl-D-aspartate (NMDA) receptor is known to be most importantly involved and ROS plays an important role in NMDA receptor phosphorylation [5]. Phosphorylation of the NMDA receptor subunit 1 (NR1), another important component of the NMDA receptor, plays another important role in central sensitization [6].
In recent animal studies, Phenyl N-Tert-butylnitrone (PBN), a spine-trapping agent that blocks free radicals, illustrated its pharmacologic effects by inhibiting cell damage resulting from ischemia-reperfusion injury and endotoxin shock. This antiseptic action is thought to suppress the production of various pro-inflammatory genes through methods that include free radical-scavenging mechanisms [7], thus showing strong antinociceptive and pain preventative effects [3].
In our study, pain was provoked by injecting formalin into a rat's hind paw. The occurrence of pain was divided into 2 phases based on time: Phase 1 reflected acute inflammatory response to pain while phase 2 reflected tonic pain due to central stimulation [8]. There are many studies regarding ROS: the chronic constriction injury (CCI) models, heat hyperalgesia, spinal ligation models, and capsaicin-induced pain models. However, very few studies have been carried out on central sensitization of pain through the formalin test. Therefore, this study was performed to define the effects of ROS on central sensitization and its degree of manifestation on the spinal cord. We also compared the effects of PBN, an ROS scavenger, on acute pain and continuous pain due to central sensitization when injected systematically and intrathecally.
Male Sprague-Dawley rats, weighing 240-300 g each, were divided into plastic containers containing clean sawdust, 3 or less in each container, and were allowed to feed freely on food and water. The breeding room was controlled with light every 12 hours and maintained at a constant temperature (approximately 23℃) and humidity (approximately 50%). All experiments followed the rules approved by the Medical School's Animal Experiment Committee.
The rats were first divided into the intraperitoneal (IP) group and the intrathecal (IT) group, and once again divided into an experimental group and a control group. In the IP group, the formalin test was performed after intraperitoneally injection of normal saline in the control group (n = 10). The experimental group of the IP group was once again divided into 3 groups, IP 20 (n = 10), IP 50 (n = 10), and IP 100 (n = 10). The formalin test was performed after intraperitoneal injection of 20 mg/kg, 50 mg/kg, and 100 mg/kg of PBN, respectively. In the IT group, the formalin test was performed after intrathecal injection of normal saline in the control group (n = 10). The experimental group of the IT group was once again divided into 2 groups, IT 3 (n = 10) and IT 10 (n = 10). The formalin test was performed after intrathecal injection of 0.3 mg and 1.0 mg of PBN, respectively.
A transparent semicircular plastic container slightly larger than the animal was placed above the metal net in order to easily observe the rats' hind paw. They were given 40 minutes to adapt to the plastic container prior to intraperitoneal injection. Rats being intrathecally injected were also given 40 minutes to adapt to the plastic container prior to injection. Intrathecal injection was performed 20 minutes before the formalin test.
All rats were subcutaneously injected with 50 µl of 5% formalin in the left hind paw with a 25 G needle attached to a syringe. The injected foot was then observed every 5 minutes for 60 minutes for spontaneous flinching, which was observed in 2 separate phases, phase 1 representing time from formalin injection into the hind foot to 5 minutes past injection, while phase 2 represented 5 to 60 minutes after injection.
Since sedation or anesthesia can affect pain behaviors, additional tests were performed in order to distinguish whether PBN induced sedation or anesthesia. Because sedation or anesthesia interferes with posture and righting reflexes, we used a 5-point scale:
Five-point scale for posture
Five-point scale for righting reflexes
A solution of phenyl N-tert-butylnitrone (PBN, molecular weight, 177.24, Sigma Chemical Company) dissolved in normal saline was used. In order to inject a total of 5 ml/kg, a concentration of 4 mg/ml, 10 mg/ml, and 20 mg/ml were made to be given 20 mg/kg, 50 mg/kg, and 100 mg/kg, respectively. The control group, however, was injected with only 5 ml/kg of normal saline. A dose of 1 mg and 0.3 mg, 50 µl each, was injected intrathecally with an additional 10 µl of normal saline into the catheter in order to flush the remaining drug in the catheter. Therefore, the control group was injected with an overall total of 60 µl of normal saline.
After inducing general anesthesia with enflurane, the rat's fur was removed from the incision site and the body site was sterilized with potadine. After making a midline skin incision from the forth lumbar vertebrae (L4) to the second sacral vertebrae (S2), the muscles were extracted and the L6 spinous process was removed. A 20 G needle was used as a guide for dura puncture. A prepared catheter (sterilized PE 10 tube) was inserted into the 5th and 6th interlamina space, placing it in the lumbar enlargement. The catheter was then fixed behind the neck and the skin was sutured. After making sure neurologic signs were intact after awakening, PBN was injected 4 days later. Subsequent to termination of all experiments, 50 µl of methylene blue was injected into the catheter in order to identify and confirm the exact location of the catheter in the spinal cavity. We confirmed that all catheters were placed in the lumbar enlargement.
After inducing pain by injecting formalin into the rat's hind paw, immunochistochemistry testing was performed to assess nitrotyrosine, an oxidative stress marker, to identify the degree of protein nitration. After injecting normal saline or PBN, either intraperitoneally (100 mg/kg) or intrathecally (1 mg), the formalin test was performed and the rats were observed for 60 minutes. After anesthetizing the rat with ether, the tissue was fixated. Lumbar enlargement was removed and cross sectioned. The sections were incubated with anti-nitrotyrosine (a peroxynirite marker; 1 : 100; Sigma Chemical), followed by secondary antibodies conjugated with fluorescent dyes, Alexa Fluor 568 (red; Invitrogen, Eugene, Oregon, USA). A positive control for nitrotyrosine immunostaining was prepared with antinitrotyrosine antibody for cord sections of normal rats. The spinal cord was examined and photographed with a × 100 objective microscope.
All values were displayed as means ± standard error of the mean (SEMs) and analyzed with SPSS (Version 17 Predictive Analytics Software Statistics). Statistical analyses were done using one-way analyses of variance (ANOVAs), 2-way repeated ANOVAs follow by Tukey's multiple comparison test. Data was regarded to be statistically significant only when the P value was < 0.05.
Both groups displayed biphasic pain responses. In the intraperitoneal PBN group, Group IP 100 showed statistically significant decrease in the number of flinches in phase 1 and 2. Group IP 50 showed statistically significant decrease in phase 1 and 25 minutes later in phase 2. Group IP 20 showed statistically significant decrease in phase 1 and 35, 40, 45, and 55 minutes later in phase 2 compared to the control group (Fig. 1). In the intrathecal PBN group, Groups IT 3 and IT 10 showed statistically significant decreases in the number of flinches in phase 1 and 20 minutes later in phase 2. Also, intrathecally injected dose-dependent PBN reduced pain behaviors in phase 1 and 2 (Fig. 2).
Sedation due to PBN injection may alter nociceptive reactions. The 5-point scale for posture and righting reflexes after injection of the drug was 0. Changes in pain behavior were thought to be due to analgesia rather than the sedation effects of PBN.
Immunohistochemical staining of nitrated proteins was nearly undetectable in normal rats. Immunohistochemical staining in saline pre-treated rats revealed an increase in nitrated proteins in the gray matter of the lumbar spinal cord while significant decrease was observed in PBN pretreated rats.
Nociceptive behavior in phase 1 and 2 in the formalin test decreased when PBN, a strong ROS scavenger was intraperitoneally injected, illustrating that ROS is deeply involved in acute and tonic pain. Intraperitoneal PBN injection induced systemic absorption, affecting peripheral, spinal, and cerebral areas, inhibiting ROS action resulting in analgesia. Similarly, the intrathecally injected group displayed analgesic reactions in phases 1 and 2. The 50 µl of methylene blue was injected through the intrathecal catheter to localize the spinal cavity. The major area responsible for analgesic reaction of intrathecal PBN is thought to be the spine rather than the peripheral or cerebral areas.
Proinflammatory, pronociceptive mediators such as bradykinin (BK), serotonin, histamine, nitric oxide, prostaglandins (PGs), and cytokines produced by tissue damage, cause acute hyperalgesia. Cytokine secretion from spinal cord glial cells and other inflammatory cells induce pain [9]. ROS activates redox-sensitive transcription factors, such as nuclear factorkB and activator protein-1 produce various proinflammatory, pronociceptive cytokines [10,11]. Increased glutamate activates NMDA receptors to allow inflow of calcium into the cell, causing mitochondria overload in calcium, destabilizing mitochondr ia membrane potential and accelerating mitochondrial electron transport, thus increasing production of ROS [12]. Therefore, increases in spinal ROS are due to increase in mitochondrial superoxide [13]. According to recent studies, changes in glutamate transporter activity are involved in the pathophysiology of neuropathic pain [14,15]. Glutamate is the most important excitatory neurotransmitter in the central synapse, and the glutamate transporter plays an important role in maintaining low homeostatic micromolar levels of extracellular glutamate. ROS inhibits the function of glutamate transporters and activates NMDA receptors [16], inducing increases in pain as a result of the activation of excitatory neurotransmitter glutamate receptors.
An increase of ROS in nerve cells is due to nitration and inactivation of ROS, leading to a decrease in manganese superoxide dismutase (MnSOD) [17,18]. Activation of the NMDA receptor increases superoxide and nitric oxide, as well as peroxynitrite formed from when reacting with one another [19-21]. Peroxynitrite has various proinflammatory cytotoxic effects [22,23] and is thought to be responsible for the hyperalgesic effects of superoxide [24]. Increased superoxide in the spinal cord level reacts with NO to form peroxynitrite, once again converting to nitrate, inactivating MnSOD thus increasing ROS and inducing pain [24]. Superoxide and peroxynitrite activates poly ADP ribose polymerase (PARP) depleting nicotinamide adenine dinucleotide (NAD+) and diminishing intracellular ATP, causing cellular damage [25,26]. When injecting drugs through similar mechanisms of superoxide dismutase (SOD), hyperalgesia induced by inflammatory mediator substance injection such as carrageenan was reduced [24]. Thus, continuous stimulation and inflammation can cause oxidative stress to the nerve cell, thereby increasing protein nitration. In the immunohistochemical staining of this experiment, pre-treatment with PBN prevents protein nitration, an oxidative stress marker. Therefore, protein nitration in nerve cells plays an important role in central sensitization.
NMDA receptor phosphorylation is significantly increased in the dorsal areas of the spine in neuropathic pain and capsaicin induced hyperalgesia [6,27]. This is mainly due to phosphorylation of NMDA receptor subunit 1 (pNR1) distribution of protein kinase A, and focalized protein kinase C [15]. ROS activates adenyl cyclase that increases cAMP, thereby activating protein kinase [28,29]. Thus, phosphorylation of NMDA receptors by ROS is important in central sensitization and the continuous provocation of pain. Removal of ROS by administrating PBN decreases the manifestation of pNR1 in the dorsal area of the spine in neuropathic rats [14].
PBN possesses a neuroprotective property and by acting as a potent free-radical scavenger, it interferes with gene induction and NFkB activation induced by inducible NOS (iNOS) [7]. PBN has strong analgesic reactions to neuropathic pain and is proven to prevent pain. Analgesic reactions are reported in spinal nerve ligation, formalin-induced pain, and zymason-induced visceral pain [3,14]. Kim et al. [30], used a spinal nerve ligation model by systemically injecting PBN to decrease analgesic behavior. They reported that PBN did not change numbers of ectopic discharges of the dorsal root ganglia or peripheral nerves, but presented strong analgesic reactions, indicating that the spine was responsible for the actions of intrathecal PBN. Accelerated pain caused by acute pain and central sensitization was decreased according to the dosage of intraperitoneal or intrathecal administration of PBN. Systemic absorption following administration of intraperitoneal PBN to the brain, spinal cord, and liver took 20 minutes, while 134 minutes were consumed in half elimination of blood concentration. Therefore, PBN was administered 40 minutes before the formalin test. Localization by injecting 50 µl of methylene blue through a catheter into the spinal cavity allowed us to assume PBN would affect the spinal cords. In conclusion, intraperitoneally and intrathecally injected groups both displayed significant decrease in analgesic behavior, therefore presuming that the main analgesic action location of PBN was the spinal cord. However, further studies must be pursued to validate this presumption.
In summary, intraperitoneal and intrathecal administration of PBN decreased analgesic behaviors in rats in the formalin test. ROS is thought to be mainly responsible for acute pain and central sensitization. We can expect ROS scavengers, such as PBN to be excellent analgesics in refractory pain in the future.
References
1. . FY 95 funding mechanisms for the national action plane on breast cancer. NIH Guide 1995; 24: 1-15.

2. Zimmermann H. Pathobiology of neuropathic pain. Eur J Pharmacol 2001; 429: 23-37. PMID: 11698024.


3. Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 2004; 111: 116-124. PMID: 15327815.


4. Kim HK, Kim JH, Gao X, Zhou JL, Lee I, Chung K, et al. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain 2006; 122: 53-62. PMID: 16524661.


5. Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain 2007; 131: 262-271. PMID: 17317010.



6. Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain 2005; 116: 62-72. PMID: 15936881.


7. Kotake Y. Pharmacologic properties of phenyl N-tert-butylnitrone. Antioxid Redox Signal 1999; 1: 481-499. PMID: 11233146.


8. Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain 1996; 64: 345-355. PMID: 8740613.


9. Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci 2001; 24: 450-455. PMID: 11476884.


10. Gius D, Botero A, Shah S, Curry HA. Intracellular oxidation reduction status in the regulation of transcription factors NF-kappa B and AP-1. Toxicol Lett 1999; 106: 93-106. PMID: 10403653.


11. Matata BM, Galinanes M. Peroxynitrite is an essential component of cytokine production mechanism in human monocytes through modulation of nuclear factor-kappa B DNA binding activity. J Biol Chem 2002; 277: 2330-2335. PMID: 11706022.


12. Pereira CF, Oliveria CR. Oxidative glutamate toxicity involves mitochondrial dysfunction and perturbation of intracellular Ca2+ homeostasis. Neurosci Res 2000; 37: 227-236. PMID: 10940457.


13. Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain 2008; 138: 514-524. PMID: 18375065.



14. Binns BC, Huang Y, Goettl VM, Hackshaw KV, Stephens RL Jr. Glutamate uptake is attenuated in spinal deep dorsal and ventral horn in the rat spinal nerve ligation model. Brain Res 2005; 1041: 38-47. PMID: 15804498.


15. Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci 2003; 23: 2899-2910. PMID: 12684477.



16. Hacimuftuoglu A, Handy CR, Goettl VM, Lin CG, Dane S, Stephens RL Jr. Antioxidants attenuate multiple phases of formalin-induced nociceptive response in mice. Behav Brain Res 2006; 173: 211-216. PMID: 16919817.


17. Yamakura F, Taka H, Fujimura T, Murayama K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem 1998; 273: 14085-14089. PMID: 9603906.


18. MacMillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res 2001; 34: 325-336. PMID: 11328670.


19. Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature 1993; 364: 535-537. PMID: 7687749.



20. Kitto KF, Haley JE, Wilcox GL. Involvement of nitric oxide in spinally mediated hyperalgesia in the mouse. Neurosci Lett 1992; 148: 1-5. PMID: 1284437.


21. Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implication for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 1990; 87: 1620-1624. PMID: 2154753.



22. Squadrito GL, Pryor WA. The formation of peroxynitrite in vivo from nitric oxide and superoxide. Chem Biol Interact 1995; 96: 203-206. PMID: 7728908.


23. Salvemini D, Jensen MP, Riley DP, Misko TP. Therapeutic manipulations of peroxynitrite. Drug News Perspect 1998; 11: 204-214. PMID: 15616662.

24. Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, et al. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther 2004; 309: 869-878. PMID: 14988418.


25. Szabó C, Dawson VL. Role of poly (ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol Sci 1998; 19: 287-298. PMID: 9703762.


26. Inoue S, Kawanishi S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett 1995; 371: 86-88. PMID: 7664890.


27. Zou X, Lin Q, Willis WD. Enhanced phosphorylation of NMDA receptor 1 subunits in spinal cord dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. J Neurosci 2000; 20: 6989-6997. PMID: 10995844.



28. O'Flaherty C, de Lamirande E, Gagnon C. Phosphorylation of the arginine-X-X-(serine/threonine) motif in human sperm proteins during capacitation: modulation and protein kinase A dependency. Mol Hum Reprod 2004; 10: 355-363. PMID: 14997001.



29. O'Flaherty C, de Lamirande E, Gagnon C. Positive role of reactive oxygen species in mammalian sperm capacitation: triggering and modulation of phosphorylation events. Free Radic Biol Med 2006; 41: 528-540. PMID: 16863985.


30. Kim HK, Zhang YP, Gwak YS, Abdi S. Phenyl N-tert-butylnitrone, a free radical scavenger, reduces mechanical allodynia in chemotherapy-induced neuropathic pain in rats. Anesthesiology 2010; 112: 432-439. PMID: 20068451.


Fig. 1
Time response curve for the number of flinches in the formalin test after intraperitoneal injection of PBN. Intraperitoneal PBN (20 mg/kg, 50 mg/kg, and 100 mg/kg) diminished nociceptive behaviors significantly during the first and second phase. Data are presented as number of flinches. Each point on the graph represents mean ± SEM. PBN: Phenyl N-tert-butylnitrone. *P < 0.05, PBN 100 mg/kg compared with the control group. †P < 0.05, PBN 50 mg/kg compared with the control group. ‡P < 0.05, PBN 20 mg/kg compared with the control group.
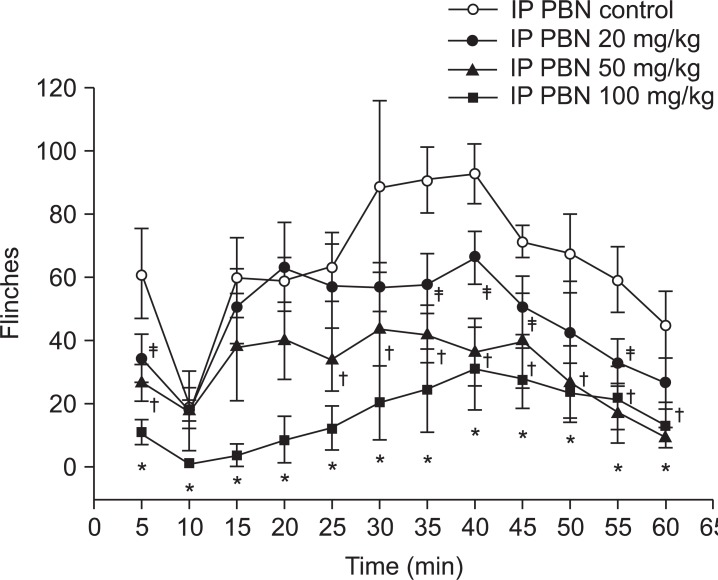
Fig. 2
Time response curve for the number of flinches in the formalin test after intrathecal PBN injection. Intrathecal PBN (0.3 mg/kg, 1 mg/kg) diminished nociceptive behaviors significantly during the first and second phase. Data are presented as number of flinches. Each point on the graph represents mean ± SEM. PBN: Phenyl N-tert-butylnitrone. *P < 0.05, IT PBN 1 mg compared with control group. †P < 0.05, IT PBN 0.3 mg compared with control group.

Fig. 3
Immunohistochemistry of nitrotyrosine lumbar dorsal horn. Immunohistochemical staining of nitrated proteins was nearly undetectable in normal rats. Immunohistochemical evaluation of saline pre-treated rats reveal increased nitrated proteins in the gray matter of the lumbar spinal dorsal horn, and a significant decrease in intraperitoneal or intrathecal PBN pretreated rats. PBN: Phenyl N-tert-butylnitrone.

Fig. 4
Immunohistochemistry of nitrotyrosine lumbar ventral horn. Immunohistochemical staining of nitrated proteins was nearly undetectable in normal rats. Immunohistochemical evaluations of saline pre-treated rats reveal increased nitrated proteins in the gray matter of the lumbar spinal ventral horn and significant decrease in intraperitoneal or intrathecal PBN pre-treated rats. PBN: Phenyl N-tert-butylnitrone.

- TOOLS








