 |
 |
|
|
Abstract
We report a rare case of a 72-year-old female who developed extensive subcutaneous emphysema, bilateral pneumothoraces, pneumomediastinum, pneumoperitoneum, and pneumoretroperitoneum after a percutaneous dilatational tracheostomy. The patient's T-cannula was accidentally connected to the oxygen line with a non-perforated connector. The patient rapidly developed respiratory insufficiency and subcutaneous emphysema in the neck and both shoulders. The bilateral pneumothoraces were managed using a chest tube. CT scans of the chest, abdomen, and pelvis revealed an extensive distribution of air throughout the chest and abdomen. The patient was treated successfully with supportive care. This case illustrates the rare occurrence of air passing into multiple body compartments, highlighting the potentially serious complications of a tracheostomy and the importance of intensive care during the recovery period.
Percutaneous tracheostomy, a common surgical procedure in the field of otolaryngology, is associated with complications, such as bleeding, infection, subcutaneous emphysema, pneumothorax, recurrent laryngeal nerve injury and tracheal ring fracture [1]. We encountered an rare case of extensive subcutaneous emphysema, bilateral pneumothoraces, pneumomediastinum, pneumoperitoneum, and pneumoretroperitoneum in a single individual caused by the accidental insufflation of oxygen through the tracheostomy tube site with a non-perforated connector. This case illustrates the dramatic results of such insufflation and the possible pathways of communication between the pleural space, mediastinum, peritoneal cavity, retroperitoneal space and subcutaneous tissue. Informed consent to publish this case report was obtained from the patient. We report this case with a review of the relevant literature.
A 72-year-old woman (72 kg, 158 cm) was admitted to hospital with dyspnea and hoarseness. She had been experiencing these symptoms for a year since she had undergone a thymectomy to remove a thymoma. In the hospital, she was diagnosed with bilateral vocal cord paralysis and was scheduled to undergo a percutaneous dilatational tracheostomy with a right laser cordectomy using a suspension laryngoscope under general anesthesia. Her vital signs were stable, she had no current medical problems, and her laboratory test values were within the normal limits. The preoperative chest radiograph was unremarkable except for evidence of previous surgery. She was a non-smoker with was no history of asthma, chronic obstructive lung disease or pneumothoraces.
Before the induction of anesthesia, the patient's blood pressure, heart rate and SpO2 was 130/80 mmHg, 80 beats/min and 98%, respectively. The patient was induced with pentothal sodium 5 mg/kg and succinylcholine 1 mg/kg in preparation for a potentially difficult intubation. A 6.5-mm reinforced endotracheal tube was inserted successfully with a stylet on the first attempt. Anesthesia was maintained with air 1 L/min, O2 1 L/min, sevoflurane 1.5-3 vol%, and rocuronium. During the procedure, the end tidal CO2 was maintained at approximately 30-35 mmHg, peak airway pressure was < 20 cm H2O, and SpO2 approximately 97-99%. Her vital signs, airway pressures, and end-tidal CO2 remained stable during surgery. The total time under anesthesia was two hours; the operative time was 90 minutes. There was minimal blood loss during the surgery, and the patient received a total of 800 ml Lactated Ringer's solution. The procedure was performed as planned. The patient was extubated after confirming full recovery of motor function as tested by the hand grip strength and head control. At the time of extubation, a T-cannula was inserted through the stoma site, and the patient was encouraged to breathe through the cannula. After confirming that the patient was stable, she was transferred to the recovery room.
Her vital signs first recorded in the recovery room were a blood pressure, heart rate and SpO2 of 130/80 mmHg, 85 beats/min and 99%, respectively. Approximately two minutes after arrival, her blood pressure decreased to 70/50 mmHg, heart rate increased to 140 beats/min and SpO2 dropped to approximately 65%. The patient experienced severe dyspnea and appeared cyanotic. Blood oozing from the tracheostomy site had saturated the gauze dressing. A physical examination revealed decreased bilateral breath sounds and diffuse crepitus around the neck and shoulder, suggesting subcutaneous emphysema. 10 mg ephedrine was injected to increase her blood pressure, and an arterial line was inserted through the right radial artery for continuous blood pressure monitoring. Initially, a perforated endotracheal connector (Fig. 1B) was attempted because a T-piece was not available at that time. But the connector was not perforated (Fig. 1A). As the connector was changed with perforated piece immediately after the patient's vital signs and SpO2 became poor, the oxygen supply through this closed connector would be maintained for approximately one minute at 5 L/min. The results of the first arterial blood gas analysis approximately three minutes after changing the connector were as follows: pH 7.342, PaCO2 49.0 mmHg, PaO2 87.7 mmHg, HCO3- 26.0 mmol/L, SaO2 94.3%, and bicarbonate -0.2 mmol/L. To identify the potential focus of bleeding that might have been the cause of the airway obstruction, the T-cannula was removed temporarily and suction was applied after confirming the stabilization of her vital signs and SpO2 but only a small amount of blood-tinged secretions was removed. The otolaryngologist examined the stoma site with a portable bronchoscope and found no complications related directly to the tracheostomy. At this point, the cause of deterioration of patient's condition was attributed to the closed endotracheal connector. A portable chest radiograph was requested to check for a potential pneumothroax. The portable chest radiograph revealed mild to moderate bilateral pneumothoraces as well as extensive subcutaneous emphysema over both shoulders, supraclavicular area, and left chest wall (Fig. 2A). The radiograph also showed both pneumomediastinum and pneumoperitoneum. A thoracic surgeon was called to the bedside, where he inserted a chest tube into the left chest wall to treat the more extensive pneumothorax. After chest tube insertion, the patient's vital signs became more stable, her mental status was alert, and she could follow verbal commands. The follow-up chest radiograph revealed improvement in the left-sided pneumothorax (Fig. 2B). The results of the arterial blood gas analysis performed 30 minutes after the chest tube insertion were as follows: pH 7.353, PaCO2 45.1 mmHg, PaO2 170.0 mmHg, HCO3- 24.7 mmol/L, SaO2 98.9%, and bicarbonate 1.0 mmol/L.
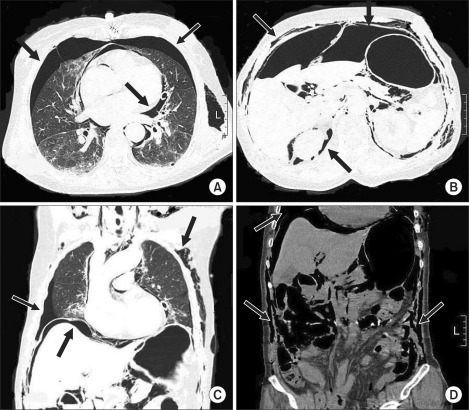
CT scans of the chest, abdomen and pelvis were performed for a further evaluation, revealing extensive subcutaneous emphysema over the chest and abdominal walls, bilateral pneumothoraces, pneumomediastinum, massive pneumoperitoneum, and pneumoretroperitoneum (Fig. 3). An MRI of the brain was performed to evaluate the damage caused by the air embolism but no specific abnormalities were found. Despite the extensive air distributed throughout the patient's trunk, the general and thoracic surgeons jointly decided to treat her conservatively.
The patient was transferred to the ICU for careful monitoring and supportive care where she remained for a week; the chest tube was left in place for ten days. Follow-up radiographs of the chest and abdomen revealed near-complete resolution of the pneumothorax, pneumomediastinum, and pneumoperitoneum. Given these findings and the cessation of symptoms, the patient was discharged two weeks after her tracheostomy. The follow-up chest radiograph three months after discharge showed no further evidence of pneumothorax or pneumoperitoneum.
Percutaneous tracheostomy involves several potential complications. The incidence of subcutaneous emphysema has been reported to be 1.4% and that of a pneumothorax to be 0.8% for percutaneous tracheostomy [2]. On the other hand, the occurrence of subcutaneous emphysema, pneumothorax, pneumomediastinum, pneumoperitoneum and pneumoretroperitoneum in the same individual is extremely rare. Although there are several such case reports [3,4], the incidence of this situation has not been reported. The other cases were associated with bowel perforation during endoscopic retrograde cholangiography and with an accidental insufflation of compressed air [3]. Both cases were managed conservatively, and the patients recovered without surgical intervention. There are many proposed mechanisms for emphysema and pneumothorax after percutaneous tracheostomy. They included damage to the anterior [5] or posterior tracheal walls [6], complications related to using a fenestrated cannula [7], cannula dislocation, paratracheal placement, and barotraumas [5,7]. These mechanisms lead to air leakage from the trachea into the subcutaneous tissue, which then follows the path of least resistance.
The present case was not a procedure-related complication of percutaneous tracheostomy itself. The complication was attributed to a misconnection of T-cannula to the oxygen line with a non-perforated connector after the otolaryngologist examined the inner tracheal lumen of the tracheostomy site, surgical wound and bronchial tree thoroughly, but found no abnormalities. The accidental connection with a closed connector at the recovery room initially lead to an insufflation of 5 L/min oxygen into the patient's lung for approximately one minute, which might have caused the volutrauma to the lung. This may have led to bilateral pneumothoraces, followed by air penetration into several body compartments, including the subcutaneous tissue, mediastinum, peritoneum, and retroperitoneum. Despite the extensive dissemination of air into multiple body compartments, the patient recovered without complications after a closed thoracostomy and conservative care in the ICU. This case illustrates the dramatic results of direct insufflation of the lungs for a relatively short time.
The airway obstruction may have been caused by a blood clot contributed to the pneumothorax [2]. Postoperative bleeding was observed at the tracheostomy site, into which blood may have been aspirated, causing an airway obstruction. An obstructing blood clot can act as a one-way valve, resulting in high airway pressures and subsequent pneumothorax. On the other hand, the bronchoscopic examination performed by the otolaryngologist in the recovery room revealed no definite abnormalities, reducing the plausibility of this explanation. Nevertheless, he may have failed to examine the entire bronchial tree, leaving the possibility of a partial airway obstruction that contributed to the development of a pneumothorax.
This case illustrates the possible pathways of communication between the pleural space, mediastinum, peritoneal cavity and retroperitoneal space. A rupture of the alveolar wall or small bronchi allows air into the mediastinum along the bronchoalveolar bundle [8] and directly into the thoracic cavity. Although the thoracic and peritoneal cavities are separated by the diaphragm, they may communicate through congenital defects, such as a pleuroperitoneal canal [9] or defects adjacent to the aorta or esophagus. Air trapped in the pleural space or the mediastinum can travel to the retroperitoneum along the tissue planes [10]. Air in the peritoneal cavity can travel to the retroperitoneal space along the mesenteric roots and vessels [3]. This type of injury also carries the risk of orbital emphysema, which can injure the optic nerve [11]. By understanding the dynamic anatomical communications between the body cavities, one can avoid an unnecessary laparotomy in the instance of pneumothorax, pneumomediastinum, pneumoperitoneum, and pneumoretroperitoneum resulting from non-surgical injuries, such as the present case.
An MRI scan of the brain was performed to rule out any brain injury or cerebral air embolism associated with the pneumothorax. A cerebral arterial air embolism can develop after a pneumothorax [12]. Fortunately, our patient escaped any hypoxic brain injury or neurological sequela despite the hypoxic episode (SpO2 < 70%) that lasted for approximately three minutes.
Although the treatment of pneumomediastinum is controversial, supportive care is the mainstay, which includes bed rest, reassurance, pain control and no oral intake to prevent esophageal rupture [13]. The treatments for pneumoperitoneum and pneumoretroperitoneum are also mainly supportive except in cases with obvious peritonitis or sepsis [14,15].
In conclusion, we experienced a rare case of air passage into multiple body compartments. This case highlights the potentially serious complications of percutaneous tracheostomy as well as the need for intensive supportive care during recovery.
References
1. Sviri S, van Heerden PV, Samie R. Percutaneous tracheostomy--long-term outlook, a review. Crit Care Resusc 2004; 6: 280-284. PMID: 16556108.


2. Fikkers BG, van Veen JA, Kooloos JG, Pickkers P, van den Hoogen FJ, Hillen B, et al. Emphysema and pneumothorax after percutaneous tracheostomy: case reports and an anatomic study. Chest 2004; 125: 1805-1814. PMID: 15136394.


3. Muramori K, Takahashi Y, Handa N, Aikawa H. Subcutaneous emphysema, pneumomediastinum, pneumothorax, penumoperitoneum, and pneumoretroperitoneum by insufflation of compressed air at the external genitalia in a child. J Pediatr Surg 2009; 44: E5-E8. PMID: 19361622.
4. Garcia P, Pizanis A, Massmann A, Reischmann B, Burkhardt M, Tosounidis G, et al. Bilateral pneumothoraces, pneumomediastinum, pneumoperitoneum, pneumoretroperitoneum, and subcutaneous emphysema after thoracoscopic anterior fracture stabilization. Spine (Phila Pa 1976) 2009; 34: E371-E375. PMID: 19404168.


5. Fikkers BG, van Heerbeek N, Krabbe PF, Marres HA, van den Hoogen FJ. Percutaneous tracheostomy with guide wire dilating forceps technique : presentation of 171 consecutive patients. Heak Neck 2002; 24: 625-631.

6. Trottier SJ, Hazzard PB, Sakabu SA, Levine JH, Troop BR, Thomson JA, et al. Posterior tracheal wall perforation during percutaneous dilatational tracheostomy: an investigation into its mechanism and prevention. Chest 1999; 115: 1383-1389. PMID: 10334157.


7. Fikkers BG, Briede IS, Verwiel JM, van den Hoogen FJ. Percutaneous tracheostomy with the Blue Rhino technique: presentation of 100 consecutive patients. Anaesthesia 2002; 57: 1094-1097. PMID: 12392457.


8. Siegel S. Case of the season. Pneumoperitoneum secondary to barotraumas. Semin Roentgenol 1994; 29: 318-320. PMID: 7809645.


9. Gattone VH 2nd, Morse DE. Pleuroperitoneal canal closure in the rat. Anat Rec 1984; 208: 445-460. PMID: 6721236.


10. Harkin CP, Summerhaug EW, Mayer KL. An unexpected complication during laparoscopic herniorrhapy. Anesth Analg 1999; 89: 1576-1578. PMID: 10589653.

11. Buckley MJ, Turvey TA, Schumann SP, Grimson BS. Orbital emphysema causing vision loss after a dental extraction. J Am Dent Assoc 1990; 120: 421-422. PMID: 2181013.


12. Cavadore P, Brunat G, Perrigault PF, Colson P. Cerebral air embolism associated with pneumothorax in a patient with pressure support ventilation. Ann Fr Anesth Reanim 2000; 19: 249-252. PMID: 10836109.


13. Gunluoglu MZ, Cansever L, Demir A, Kocaturk C, Melek H, Dincer SI, et al. Diagnosis and treatment of spontaneous pneumomediastinum. Thorac Cardiovasc Surg 2009; 57: 229-231. PMID: 19670118.



14. Brill SE, Skipworth J, Stoker DL. Conservative management of pneumatosis intestinalis and massive pneumoperitoneum in the acute abdomen: a case report. Ann R Coll Surg Engl 2008; 90: W11-W13. PMID: 18325200.



15. Ruiz-Tovar J, Lopez-Quindos P, Morales V, Martinez-Molina E. Pneumoretroperitoneum secondary to colonoscopy. Am Surg 2010; 76: 112-113. PMID: 20135953.


Fig.┬Ā1
This figure shows the oxygen line connectors used in this case. On the left side (A) is the mistakenly used non-perforated connector used in the recovery room; the perforated connector is on the right (B). A perforated connector or T-piece should have been used.

Fig.┬Ā2
A portable chest radiograph (A) shows bilateral pneumothoraces, pneumomediastinum, pneumoperitoneum, and subcutaneous emphysema over both chest walls, neck, and shoulder. Follow-up chest radiograph (B) after the left-sided closed thoracostomy revealed slight improvement in the pneumothorax.

Fig.┬Ā3
Transverse and coronal CT images of the chest, abdomen, and pelvis. A large amount of the air is visible in the pleural cavity (A, C), mediastinum (A), peritoneal cavity (B, C), retroperitoneum (B) and subcutaneous tissue (B-D). These images show the passage of accidentally insufflated oxygen into multiple body compartments, which is a serious reminder of the potential complications of tracheostomy.
- TOOLS








