|
 |
|
|
Abstract
The implantation of spinal cord stimulators (SCSs) to treat chronic intractable pain is steadily increasing. And there is an increased likelihood of instances where other therapies or procedures are found to interfere with SCS function, which in turn may result in pain. Since SCS utilize electric impulses as well as magnets, special considerations need for patients with a SCS in situ who require these procedures. The present report describes a case where radiofrequency (RF) ablation of the third occipital nerve resulted in spontaneous activation of a cervical SCS device.
Electrical spinal neuromodulation using spinal cord stimulation (SCS) devices is currently used to treat chronic pain conditions refractory to conservative treatment, including complex regional pain syndrome (CRPS), diabetic neuropathy, postherpetic neuralgia, peripheral ischemia, and low back pain [1]. Percutaneous radiofrequency (RF) neurotomy is a palliative pain-relieving intervention that has been demonstrated to be efficacious in a formal, randomized, placebo-controlled clinical trial [2]. Although both SCS and RF neuroablation are used to treat chronic pain, few reports have described the use of RF ablation in patients fitted with SCS implants, and none has described complications arising from RF neuroablation in SCS patients. The present report describes a case where RF neuroablation applied at the level of the cervical nerve resulted in spontaneous activation of a cervical SCS device.
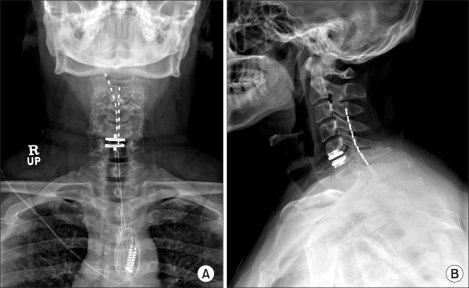
A 49-year-old male had undergone artificial disc insertion at the 5th and 6th cervical levels because of disc rupture after a car accident. Immediately after that operation, he developed severe left neck and arm pain, of VAS 10. One year postoperatively he presented to us with posterior neck and left arm pain accompanied by headache. On physical examination, the patient complained of hyperalgesia, motion limitation, weakness, and numbness. The left arm was atrophic and the hand was edematous. He was assessed as having CRPS type 2. Pain management consisted of physical therapy, analgesics, epidural steroid injection, nerve block, and ketamine injection therapy. However, intractable left arm, shoulder, and neck pain continued. The pain gradually worsened, and the pain area expanded to the right arm and both occipital areas. We decided upon neuromodulation, and he underwent surgery to insert a cervical SCS with a dual eight-contact lead (Octrode™; Eon® rechargeable implantable pulse generator system; Advanced Neuromodulation Systems, Plano, TX) (Fig. 1).
SCS insertion resulted in a 50% decrease in pain. However, he continued to experience constant shoulder, neck, and occipital area pain. The treatments used included pulsed RF of the C2 dorsal root ganglion, a third occipital nerve block, a medial branch nerve block, trigger point injection at the C4-6 level, ketamine therapy, and oral medication. All procedures were tolerable and produced relatively good results without complications.
Approximately 1 year after SCS insertion, we decided to treat the residual suboccipital and upper neck pain by RF ablation to the left third occipital nerve and left third cervical medial branch. The patient was placed in a supine position, the implantable pulse generator system (IPG) was turned off, and an RF electrode was inserted under x-ray guidance after local infiltration with 1% lidocaine. RF ablation (NeuroTherm® JK3 instrument; RDG Medical, Surrey, United Kingdom) at the third cervical medial branch was performed twice for 60 seconds at 60℃ without any complications. However, when RF ablation was performed at the left third occipital nerve, the patient suddenly complained of severe pain when the probe temperature was 40℃. The patient given administered an injection of 2 ml 1% lidocaine, and RF ablation was again attempted. Within 10 seconds, the temperature of the probe suddenly increased from 40℃ to 60℃, and the patient complained of severe pain and showed paresthesia in both hands. The procedure was immediately halted, but the patient remained in pain and parasthesia, and said he felt the same symptoms as if the SCS was on. We decided against any further RF treatment and placed the patient in a sitting position. Eventually the pain subsided. There were no changes to the prior settings of the SCS equipment. The patient was sent to a general ward in the sitting position. We rechecked the SCS device and could not find any mechanical problem. At the 1-month follow-up, the patient reported about 20% pain relief in the upper neck area, and there were no specific changes to the SCS parameter settings. Further follow-up comprised of examination in the outpatient clinic.
SCS has been established since the late 1970s as an advanced treatment for chronic intractable pain [3]. Spinal electrical neuromodulation is increasingly used, both in terms of the number of patients being treated and the number of conditions for which the procedure is indicated. Therefore, the number of patients who have SCS implants and who may also require other types of therapy, or procedures for other conditions, is increasing. For example, SCS patients may require implantation of permanent pacemakers (PPM) for arrhythmia, or may need to undergo magnetic resonance imaging (MRI). In the present case, the patient required RF ablation for the treatment of residual pain. Whereas a number of studies have reported on issues relating to PPM implantation and MRI in SCS patients, few have examined RF ablation in SCS patients.
A combination of SCS and PPM has been considered hazardous because of the risk of interdevice interference causing severe bradycardia or cardiac arrest [4,5]. However, one study concluded that bipolar SCS and PPM was a safe combination [3].
MRI is considered unsuitable or contraindicated for SCS patients according to the two largest SCS manufacturers. MRI involves three energy fields, namely a strong static magnetic field and its associated spatial gradient, a pulsed gradient magnetic field, and a pulsed radiofrequency field. Any one, or a combination, of these fields may affect SCS function [6]. The static field can affect the SCS extension cable or the IPG [7]. Tronnier and associates found that IPGs were automatically switched on or off in patients who had plate electrodes connected to an IPG, and that the turning on resulted in abrupt severe pain. In addition, the fields can induce a rotational force (torque) on the SCS device, resulting in adverse events including lead movement, lead dislodgement, and neural tissue activation [6,8]. However, several case reports have demonstrated that MRI can be safely performed on SCS patients [9-11]. A key reason is that modern implanted electrodes, extensions, and IPGs do not contain ferromagnetic materials, and are therefore not susceptible to MRI field effects [6,11].
Applying RF currents to nerves to treat pain has been practiced for more than 30 years. However, few reports have examined the safety of RF neuroablation in SCS patients, or the effect of the RF ablation electromagnetic field on the SCS system. Many pain clinicians feel confident performing RF neuroablation as their experience indicates that the procedure is safe and has no effect on SCS systems. But the present case indicates that physicians must be aware that there is a risk of complications when the RF needle is placed close to the electrode, because of an effect of the RF field on the stimulator device. The RF electromagnetic field may cause SCS device electronics to malfunction. Ruggera and colleagues [12] stated that electromagnetic energy from a diathermy applicator was collected by the conductive implant and redistributed in the form of a radiofrequency electrical current that was conducted into tissue surrounding the conductive surface of the implant. In the present case, it appears that such an electromagnetic current caused a malfunction that resulted spontaneous activation of the device. Because parameters were set with the patient in an upright position, severe pain was experienced in the supine position. Changes of posture or abrupt movements may vary the degree of stimulation and cause unpleasant sensations or pain.
In summary, we believe that great care should be taken when performing RF neuroablation on SCS patients because of possible RF field effects on the stimulator device. An RF probe placed too close to the device may generate enough electromagnetic power to interfere with neurostimulator function. However, complications are unlikely if the RF probe is positioned some distance from an IPG or electrode. In our case, no long-term adverse effects were noted. Therefore, we recommend that physicians keep an appropriate distance between the RF probe and the electrode to minimize the risk of complications. Further studies are required to better characterize the safety issues surrounding RF neuroablation in SCS patients.
References
1. Vallejo R, Kramer J, Benyamin R. Neuromodulation of the cervical spinal cord in the treatment of chronic intractable neck and upper extremity pain: a case series and review of the literature. Pain Physician 2007; 10: 305-311. PMID: 17387353.


2. Lord SM, Barnsley L, Wallis BJ, McDonald GJ, Bogduk N. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med 1996; 335: 1721-1726. PMID: 8929263.


3. Kosharskyy B, Rozen D. Feasibility of spinal cord stimulation in a patient with a cardiac pacemaker. Pain Physician 2006; 9: 249-251. PMID: 16886033.

4. Romanó M, Zucco F, Baldini MR, Allaria B. Technical and clinical problems in patients with simultaneous implantation of a cardiac pacemaker and spinal cord stimulator. Pacing Clin Electrophysiol 1993; 16: 1639-1644. PMID: 7690931.


5. Romano M, Brusa S, Grieco A, Zucco F, Spinelli A, Allaria B. Efficacy and safety of permanent cardiac DDD pacing with contemporaneous double spinal cord stimulation. Pacing Clin Electrophysiol 1998; 21: 465-467. PMID: 9507551.


6. De Andres J, Valía JC, Cerda-Olmedo G, Quiroz C, Villanueva V, Martinez-Sanjuan V, et al. Magnetic resonance imaging in patients with spinal neurostimulation systems. Anesthesiology 2007; 106: 779-786. PMID: 17413916.


7. Tronnier VM, Staubert A, Hähnel S, Sarem-Aslani A. Magnetic resonance imaging with implanted neurostimulators: an in vitro and in vivo study. Neurosurgery 1999; 44: 118-125. PMID: 9894972.


8. Finelli DA, Rezai AR, Ruggieri PM, Tkach JA, Nyenhuis JA, Hrdlicka G, et al. MR imaging-related heating of deep brain stimulation electrodes: in vitro study. AJNR Am J Neuroradiol 2002; 23: 1795-1802. PMID: 12427641.


9. Kiriakopoulos ET, Tasker RR, Nicosia S, Wood ML, Mikulis DJ. Functional magnetic resonance imaging: a potential tool for the evaluation of spinal cord stimulation: technical case report. Neurosurgery 1997; 41: 501-504. PMID: 9257323.


10. Parisod E, Murray RF, Cousins MJ. Conversion disorder after implant of a spinal cord stimulator in a patient with a complex regional pain syndrome. Anesth Analg 2003; 96: 201-206. PMID: 12505953.


11. Liem LA, van Dongen VC. Magnetic resonance imaging and spinal cord stimulation systems. Pain 1997; 70: 95-97. PMID: 9106814.


12. Ruggera PS, Witters DM, von Maltzahn G, Bassen HI. In vitro assessment of tissue heating near metallic medical implants by exposure to pulsed radio frequency diathermy. Phys Med Biol 2003; 48: 2919-2928. PMID: 14516109.


- TOOLS