|
 |
|
|
Abstract
Background
Postoperative nausea and vomiting (PONV) is the major complication related to general anesthesia, occurring in 60–80% of patients after thyroidectomy. The objective of this study was to compare the effects of an intraoperative dexmedetomidine infusion with remifentanil, as anesthetic adjuvants of balanced anesthesia, on PONV in patients undergoing thyroidectomy.
Methods
Eighty patients scheduled for thyroidectomy were randomized into the following two groups: 1) The dexmedetomidine group (Group D), who received an initial loading dose of dexmedetomidine (1 µg/kg over 10 min) during the induction of anesthesia, followed by a continuous infusion at a rate of 0.3–0.5 µg/kg/h; 2) the remifentanil group (group R), who received remifentanil at an initial target effect site concentration of 4 ng/ml during the induction of anesthesia, followed by a target effect site concentration of 2–3 ng/ml. PONV was assessed during the first 24 hours in 2 time periods (0–2 h and 2–24 h). The pain intensity, sedation score, extubation time, and hemodynamics were also assessed.
Results
During the 2 time periods, the incidence and severity of PONV in group D were significantly lower than in group R. In addition, the need for rescue antiemetics was significantly lower in group D than in group R. The effect of dexmedetomidine on postoperative pain relief (2–24 h) was superior to that of remifentanil. The hemodynamics were similar in both groups, whereas eye opening and extubation time were delayed in group D.
Postoperative nausea and vomiting (PONV) is one of the major complications related to general anesthesia, with a reported incidence as high as 60–80% after thyroidectomy [1,2,3]. Patients with PONV experience increased discomfort, electrolyte imbalances, postoperative bleeding, and aspiration of gastric contents, which can result in a delayed discharge and increase the costs of care [4]. There have been many efforts towards attenuating the incidence and severity of PONV; however, it remains a significant challenge in the field of anesthesia.
Dexmedetomidine is a highly selective α2-adrenoreceptor agonist, which has versatile effects including sedative, amnestic, analgesic, and sympatholytic properties [5,6]. In addition, the use of dexmedetomidine as an anesthetic adjuvant spares the use of intra-operative opioids and inhalation anesthetics [7]. Recently, the interest related to using dexmedetomidine as part of balanced anesthesia has been growing. Many studies have shown the efficacy of dexmedetomidine with a favorable recovery profile [8], hemodynamic stability [6], postoperative analgesia [6], and reduction of PONV [9]; however, controversy over the attenuation of PONV persists.
Generally, the combination of the inhalational anesthetic as hypnotic agent and a short acting opioid as an analgesic agent is the most popular regimen for balanced anesthesia [10]. As it clears rapidly, remifentanil is a popular choice as an anesthetic adjuvant in balanced anesthesia [11]. Opioids are well-known risk factors for developing PONV; however, the occurrence of remifentanil-related PONV is unclear, and it has actually been shown to be associated with less PONV [12,13].
Therefore, we intended to compare the effects of intraoperative dexmedetomidine infusion as an anesthetic adjuvant of balanced anesthesia on PONV with those of remifentanil in patients undergoing thyroidectomy.
This study was approved by the committee of Institutional Review Board at our hospital. We obtained the written informed consent from all patients and enrolled 80 patients, aged 18 to 60 years, with an American Society of Anesthesiologists physical status classification of I or II, who were scheduled for an elective thyroidectomy. Exclusion criteria were allergy to either dexmedetomidine or remifentanil, use of opioids or antiemetics before the operation, body mass index more than 35 kg/m2, and cognitive impairment.
Patients were allocated by computer-generated randomization to receive either dexmedetomidine (Group D, n = 40) or remifentanil (Group R, n = 40) for balanced anesthesia during anesthetic maintenance. There were no patients who received premedication. After standardized monitoring (electrocardiography, non-invasive blood pressure, pulse oximetry, and bispectral index [BIS]), anesthesia was induced with propofol (1.5 to 2.5 mg/kg) and rocuronium (0.6 to 1 mg/kg). Group D patients received an initial loading dose of dexmedetomidine (1 µg/kg over 10 min) during the induction of anesthesia, followed by a continuous infusion at a rate of 0.3–0.5 µg/kg/h throughout the operation. Group R patients received remifentanil at an initial target effect site concentration (TESC) of 4 ng/ml during the induction of anesthesia, followed by a TESC of 2–3 ng/ml throughout the operation using a target controlled infusion device (Orchestra® Base Primea, Fresenius Vial, Brezins, France). After intubation, anesthesia was maintained with a 1.0–2.5% end-tidal concentration of sevoflurane in 50% oxygen with air, a continuous infusion of dexmedetomidine or remifentanil, adjusted to maintain an acceptable hemodynamic response and a BIS of 40–60. Phenylephrine (100 µg for a systolic blood pressure [SBP] below 80 mmHg and heart rate [HR] above 50 beats/min) or ephedrine (4 mg for a SBP below 80 mmHg and HR below 50 beats/min) was administered to prevent hypotension or bradycardia. If repetitive phenylephrine injections were required, it was continuously infused. The infusion of dexmedetomidine or remifentanil was continued until the surgery was completed. Residual muscle relaxation was antagonized by intravenous (IV) pyridostigmine (0.2 mg/kg) and IV glycopyrrolate (0.01 mg/kg).
Upon arrival to the operating room, blood pressure (BP) and HR were measured at baseline (T0), just before intubation (T1), just after intubation (T2), 5 min after intubation (T3), and 10 min after intubation (T4). During emergence from anesthesia, the time to eye opening, defined as the time interval from the discontinuation of drugs (sevoflurane, dexmedetomidine, or remifentanil) to the first eye opening in response to only verbal commands without tactile stimulus, was recorded. Extubation time (from the cessation of inhalational anesthetics to the removal of the endotracheal tube when patients regain consciousness with spontaneous breathing) was also assessed.
The incidences of PONV was assessed during first 24 hours in 2 time periods (0–2 h and 2–24 h after surgery), and the severity of nausea was analyzed using a 4-point scale (0 = absent; 1 = mild; 2 = moderate; 3 = severe) during those times. Based on the study by Apfel et al. [14,15], PONV should be observed over 24 h. Since volatile anesthetics are considered to be the cause of PONV in the early period (0–2 h after surgery) with their rapid pharmacokinetics, additional reporting of early (0–2 h) and delayed (2–24 h) postoperative vomiting is desirable. When the patient requested a rescue drug or had any emetic episodes, 10 mg of IV metoclopramide was injected.
The pain intensity was assessed using the visual analog scale (VAS) ranging from 0 (no pain) to 10 (worst possible pain). When the pain score was > 4 or the patient requested rescue analgesics, 30 mg of IV ketorolac was injected. Hemodynamic responses were recorded on arrival to the PACU (T5) and then every 5 min for 20 min (T6–9) while in the PACU. A modified observer's assessment of alertness (OAA/S) score of sedation (scale of 0–5; 0 represents no response to a noxious stimulus and 5 represents being awake and responsive to their name when spoken to in a normal tone) was also recorded 5 min after arrival to the PACU [16]. Lastly, the duration of the PACU stay was assessed. All data were assessed by an anesthesiologist who was blinded to the study.
In our preliminary study, the incidence of PONV after thyroidectomy was 70% in patients receiving sevoflurane and remifentanil. It was decided that a 50% reduction in the incidence of PONV in the dexmedetomidine group would be clinically relevant and 31 patients were required per group (α = 0.05, power = 80%). Thus, 40 patients for each group were selected to increase the power, as well as to take the possible dropouts into consideration. Data were expressed as number (%) or means ± SD. Statistical analysis was performed using SPSS software (Chicago, IL, USA) and either chi-squared, Fisher's exact, t, or Mann-Whitney U tests were used as appropriate. P < 0.05 was considered statistically significant.
There was no significant difference between two groups in demographics and other characteristics (Table 1). During the 2 time periods (0–2 h and 2–24 h after surgery), the incidence of nausea in group D was significantly less than that of group R (P < 0.001 and P = 0.048, respectively). The requirement for rescue antiemetic treatments for PONV during the first 24 hours (0–2 h and 2–24 h) was significantly less in group D than in group R (P = 0.003) (Table 2). The difference in the incidence of vomiting was not significant. The severity of nausea assessed by a 4-point scale was lower in group D when compared to that of group R during the 2 time periods (0–2 h and 2–24 h after surgery) (P < 0.001 and P = 0.048, respectively) (Table 3). During the 0–2 h time period, the severity of postoperative pain assessed by VAS was not significantly different between the two groups, but during the 2–24 h time period, the postoperative VAS was lower in group D when compared to that of group R (P = 0.017). Fewer patients in group D required rescue analgesic treatments when compared to group R, but the difference did not reach statistical significance (P = 0.073) (Table 4). Eye opening and extubation time were significantly longer in group D when compared to that of group R (P < 0.001 for the both). Sedation score assessed by modified OAA/S was significantly lower in group D than in group R (P < 0.001), and PACU stay time was longer in group D than in group R (P < 0.001) (Table 4).
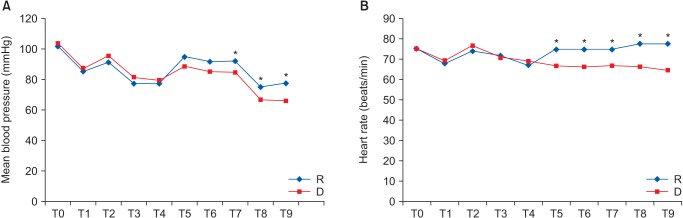
Concerning hemodynamic changes, mean blood pressure (MBP) after 10, 15, and 20 min in the PACU was significantly lower in group D. HR was significantly lower in group D on arrival and after 5, 10, 15, and 20 min in the PACU (Fig. 1). However, the differences in MBP and HR at each time point between the 2 groups were not clinically significant with regard to the administration of vasopressors or anticholinergics.
The main result of this study is that thyroidectomy patients given intraoperative dexmedetomidine as an anesthetic adjuvant had significantly less PONV during the first 24 hours postoperatively, compared to patients given remifentanil. The efficacy of dexmedetomidine for postoperative pain was also superior to remifentanil. The hemodynamic control was similar in both groups, whereas eye opening was delayed and extubation time was longer in the dexmedetomidine group.
PONV is a common and unpleasant complication after general anesthesia. The risk factors that affect the incidence of PONV are multifactorial in origin and include anesthesia, surgery, and characteristics of the patient [9]. Identifying patients who are at increased risk of PONV and providing them with prophylaxis are the main concerns of anesthesiologists and surgeons. Recently, targeting using a multimodal approach is driven by the goal of improving PONV. Many studies have demonstrated that combined antiemetic therapies are more effective than a single antiemetic treatment in patients with risk factors [17,18]. Moreover, several non-pharmacologic strategies to reduce the risk of PONV include preoperative anxiolysis, adequate hydration, and local analgesic techniques [19].
Dexmedetomidine has a unique constellation of pharmacological properties. It is only approved by the Food and Drug Administration for sedation in the intensive care setting; however, there are many clinical reports related to its off-label use as an anesthetic adjuvant during the perioperative period [8,20]. Dexmedetomidine provides perioperative hemodynamic stability, anesthesia, an opioid sparing effect, attenuation of PONV, and control of postoperative pain with minimal respiratory depression [7]. However, the preventative PONV concentration of dexmedetomidine has been unclear and dose-ranging studies for when it is infused as a balanced anesthetic technique are lacking. A meta-analysis related to the clinical usage of dexmedetomidine for PONV prevention reports that a 0.5–1.0 µg/kg bolus infusion was effective for reducing the occurrence of PONV [21]. In this study, the dexmedetomidine group that was administered a 1 µg/kg loading dose over 10 min, followed by a 0.3–0.5 µg/kg/h continuous infusion during the intra-operative period, showed better control of PONV than the remifentanil group during the first 24 h after surgery, which reduced the need for rescue antiemetics. These results could be explained by the decreased noradrenergic activity as a result of α2 presynaptic inhibition in the locus coeruleus or the reduction of sympathetic outflow, which may trigger PONV [22].
Remifentanil is a µ-opioid agonist, which has a rapid clearance and lacks complications related to its accumulation; this is because of its short half-life of 8–10 min and context-sensitive half-life of 4 min [23]. Generally, with regard to PONV, opioids are considered a risk factor; however, unlike other opioids, the effect of remifentanil-related PONV has been inconsistent in clinical situations. Morino et al. [24] identified the intraoperative use of remifentanil as a risk factor for PONV, whereas in the study by Oh et al. [25], the incidence of PONV using sevoflurane with remifentanil was not different when compared to using sevoflurane alone. In addition, Lim et al. [13] reported that the intraoperative continuous infusion of remifentanil under sevoflurane-based anesthesia did not affect PONV. Therefore, we intended to compare the effect on PONV between opioid- and opioid-free balanced anesthesia (the remifentanil and dexmedetomidine groups, respectively) and identified less postoperative nausea when the dexmedetomidine infusion was used as an anesthetic adjuvant in patients undergoing thyroidectomy. With regard to postoperative pain, remifentanil has little postoperative analgesia and may provoke hyperalgesia by activating spinal N-methyl-D-aspartate receptors [26]. In this study, the VAS scores for postoperative pain were less in the dexmedetomidine group (2–24 h) and rescue analgesic requirements were lower in the dexmedetomidine group, although it did not reach statistical significance. Our finding of reducing postoperative pain is consistent with that of Gurbet et al. [27], who demonstrated that a dexmedetomidine infusion (1 µg/kg of loading dose for 10 min followed by 0.5 µg/kg/h) during abdominal surgeries provided an effective postoperative analgesia without increasing the side effects. The possible explanation for the reduction of postoperative pain by dexmedetomidine may be related to the inhibition of substance P release from the dorsal horn by activation of the α2-adrenoreceptors, which leads to a decrease in the nociceptive inputs [28] and an elimination half-life of 2 to 2.5 hours [29]. Although the long half-life of dexmedetomidine resulted in prolonged recovery times and lower sedation scores, clinical intervention was not required.
With regard to hemodynamic stability, dexmedetomidine can cause a decrease in BP and HR by decreasing the sympathetic outflow and circulating catecholamines. However, rapid infusion of a high concentration can conversely cause an increase in BP, which may be a result of the vasoconstriction caused by the activation of the α2-adrenoreceptors. The ability of dexmedetomidine to control BP and HR has been previously reported [30]. It is important to find the therapeutic range of an anesthetic adjuvant that would maximize its various pharmacological properties, while minimizing adverse side effects such as hypotension and/or bradycardia. In this study, the occurrence of cardiovascular events requiring therapeutic intervention during the intraoperative period was not different between the two groups, which suggests that our infusion rate of dexmedetomidine (1 µg/kg of loading dose for 10 min followed by 0.3–0.5 µg/kg/h) may be appropriate as a part of balanced anesthesia for patients undergoing thyroidectomy. In the PACU, although MBP and HR tended to be lower in group D than in group R, the need for medical treatment did not differ.
In conclusion, the use of dexmedetomidine as anesthetic adjuvant provided better control of PONV and postoperative pain compared to remifentanil in patients undergoing thyroidectomy. The efficacy of hemodynamic control was similar in both groups, whereas eye opening and extubation time were delayed in the dexmedetomidine group. Therefore, the use of dexmedetomidine in the balanced anesthetic technique may be a useful approach towards achieving ideal anesthesia.
References
1. Ewalenko P, Janny S, Dejonckheere M, Andry G, Wyns C. Antiemetic effect of subhypnotic doses of propofol after thyroidectomy. Br J Anaesth 1996; 77: 463-467. PMID: 8942329.



2. Sonner JM, Hynson JM, Clark O, Katz JA. Nausea and vomiting following thyroid and parathyroid surgery. J Clin Anesth 1997; 9: 398-402. PMID: 9257207.


3. Vari A, Gazzanelli S, Cavallaro G, De Toma G, Tarquini S, Guerra C, et al. Post-operative nausea and vomiting (PONV) after thyroid surgery: a prospective, randomized study comparing totally intravenous versus inhalational anesthetics. Am Surg 2010; 76: 325-328. PMID: 20349666.


4. Golembiewski J, Chernin E, Chopra T. Prevention and treatment of postoperative nausea and vomiting. Am J Health Syst Pharm 2005; 62: 1247-1260. PMID: 15947124.


5. Wu HH, Wang HT, Jin JJ, Cui GB, Zhou KC, Chen Y, et al. Does dexmedetomidine as a neuraxial adjuvant facilitate better anesthesia and analgesia? A systematic review and meta-analysis. PLoS One 2014; 9: e93114PMID: 24671181.



6. Sun Y, Lu Y, Huang Y, Jiang H. Is dexmedetomidine superior to midazolam as a premedication in children? A meta-analysis of randomized controlled trials. Paediatr Anaesth 2014; 24: 863-874. PMID: 24666837.


7. Gupta N, Rath GP, Prabhakar H, Dash HH. Effect of intraoperative dexmedetomidine on postoperative recovery profile of children undergoing surgery for spinal dysraphism. J Neurosurg Anesthesiol 2013; 25: 271-278. PMID: 23519371.


8. Kim SY, Kim JM, Lee JH, Song BM, Koo BN. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth 2013; 111: 222-228. PMID: 23524149.



9. Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology 1992; 77: 162-184. PMID: 1609990.


10. Tonner PH. Balanced anaesthesia today. Best Pract Res Clin Anaesthesiol 2005; 19: 475-484. PMID: 16013695.


12. Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC. Remifentanil for general anaesthesia: a systematic review. Anaesthesia 2007; 62: 1266-1280. PMID: 17991265.


13. Lim H, Doo AR, Son JS, Kim JW, Lee KJ, Kim DC, et al. Effects of intraoperative single bolus fentanyl administration and remifentanil infusion on postoperative nausea and vomiting. Korean J Anesthesiol 2016; 69: 51-56. PMID: 26885302.



14. Apfel CC, Roewer N, Korttila K. How to study postoperative nausea and vomiting. Acta Anaesthesiol Scand 2002; 46: 921-928. PMID: 12190791.


15. Apfel CC, Kranke P, Katz MH, Goepfert C, Papenfuss T, Rauch S, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth 2002; 88: 659-668. PMID: 12067003.



16. Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, et al. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol 1990; 10: 244-251. PMID: 2286697.

17. Lee SJ, Lee SM, Kim SI, Ok SY, Kim SH, Park SY, et al. The effect of aprepitant for the prevention of postoperative nausea and vomiting in patients undergoing gynecologic surgery with intravenous patient controlled analgesia using fentanyl: aprepitant plus ramosetron vs ramosetron alone. Korean J Anesthesiol 2012; 63: 221-226. PMID: 23060978.



18. Riley TJ, McKenzie R, Trantisira BR, Hamilton DL. Droperidol-ondansetron combination versus droperidol alone for postoperative control of emesis after total abdominal hysterectomy. J Clin Anesth 1998; 10: 6-12. PMID: 9526930.


19. Scuderi PE, James RL, Harris L, Mims GR 3rd. Multimodal antiemetic management prevents early postoperative vomiting after outpatient laparoscopy. Anesth Analg 2000; 91: 1408-1414. PMID: 11093990.


20. Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg 2008; 106: 1741-1748. PMID: 18499604.


21. Liang X, Zhou M, Feng JJ, Wu L, Fang SP, Ge XY, et al. Efficacy of dexmedetomidine on postoperative nausea and vomiting: a meta-analysis of randomized controlled trials. Int J Clin Exp Med 2015; 8: 12113-12134. PMID: 26550123.


22. Whittington RA, Virág L. Dexmedetomidine-induced decreases in accumbal dopamine in the rat are partly mediated via the locus coeruleus. Anesth Analg 2006; 102: 448-455. PMID: 16428541.


23. Bürkle H, Dunbar S, Van Aken H. Remifentanil: a novel, short-acting, mu-opioid. Anesth Analg 1996; 83: 646-651. PMID: 8780298.


24. Morino R, Ozaki M, Nagata O, Yokota M. Incidence of and risk factors for postoperative nausea and vomiting at a Japanese Cancer Center: first large-scale study in Japan. J Anesth 2013; 27: 18-24. PMID: 22923285.


25. Oh AY, Kim JH, Hwang JW, Do SH, Jeon YT. Incidence of postoperative nausea and vomiting after paediatric strabismus surgery with sevoflurane or remifentanil-sevoflurane. Br J Anaesth 2010; 104: 756-760. PMID: 20418533.



26. Zhao M, Joo DT. Enhancement of spinal N-methyl-D-aspartate receptor function by remifentanil action at delta-opioid receptors as a mechanism for acute opioid-induced hyperalgesia or tolerance. Anesthesiology 2008; 109: 308-317. PMID: 18648240.


27. Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth 2006; 53: 646-652. PMID: 16803911.


28. Jain G, Bansal P, Ahmad B, Singh DK, Yadav G. Effect of the perioperative infusion of dexmedetomidine on chronic pain after breast surgery. Indian J Palliat Care 2012; 18: 45-51. PMID: 22837611.



29. Afonso J, Reis F. Dexmedetomidine: current role in anesthesia and intensive care. Rev Bras Anestesiol 2012; 62: 118-133. PMID: 22248773.


Fig. 1
Hemodynamic changes in both groups. T0: arrival at operation, T1: just before intubation, T2: just after intubation, T3 and T4: 5 and 10 min after intubation, T5: on arrival to the recovery room, T6, T7, T8, and T9: 5, 10, 15, and 20 min after arrival to the recovery room; mean. *P < 0.05, Group D compared to group R.