A comparison of adductor canal block before and after thigh tourniquet during knee arthroscopy: a randomized, blinded study
Article information
Abstract
Background
Adductor canal block (ACB) provides effective analgesia after arthroscopic knee surgery. However, there is insufficient data regarding whether ACB should be performed before or after inflation of a thigh tourniquet. We aimed to investigate the efficacy of ACB performed before and after placement of a thigh tourniquet and evaluate associated quadriceps motor weakness.
Methods
ACB was performed before tourniquet inflation in the PreT group, and it was performed after inflation in the PostT group. In the PO group, ACB was performed at the end of surgery after deflation of the tourniquet.
Results
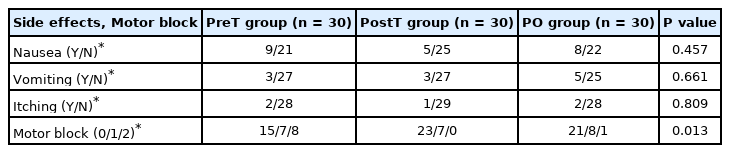
There were no statistically significant differences between the groups in terms of demographic data. There was no statistically significant difference among the three groups in terms of total postoperative opioid consumption (P = 0.513). Patient satisfaction and the amount of rescue analgesia administered were also not significantly different between the groups. There was no significant difference in terms of static and dynamic visual analog scale scores between the groups (for 24 h: P = 0.306 and P = 0.271, respectively). The incidence of motor block was higher in the PreT group (eight patients) than in the PostT group (no patients) and the PO group (one patient) (P = 0.005).
Conclusions
Using a tourniquet before or after ACB did not result in differences in terms of analgesia quality; however, applying a tourniquet immediately after ACB may lead to quadriceps weakness.
Introduction
Arthroscopic knee surgery is a routine orthopedic procedure performed to repair meniscal tears, debride/reshape cartilage flaps, and reconstruct ligaments [1-3]. Although it is a minimally invasive procedure, patients may experience moderate-to-severe pain due to port-site incision and surgical trauma to the knee ligaments [3]. Pain after arthroscopic knee surgery not only results in patient dissatisfaction but may also cause delayed mobilization. Therefore, it is important to effectively manage postoperative pain [3-5]. Analgesia after this type of surgery can be the most effective using a peripheral nerve block as part of a multimodal analgesia regimen. Peripheral nerve blocks such as femoral nerve blocks or adductor canal blocks (ACBs) may be an option for pain management [6-8]. However, motor blockade of the quadriceps muscle after a femoral nerve block may create a potential risk of falls [9].
The adductor canal is a musculoaponeurotic tunnel that functions as a passageway for neurovascular structures (such as the femoral artery, femoral vein, saphenous nerve, and nerve to the vastus medialis) from the femoral triangle to the adductor hiatus [10]. Selective blockade of the saphenous nerve in the adductor canal provides effective analgesia after surgical knee procedures [4-15]. Because the saphenous nerve is a sensory branch of the femoral nerve, its selective blockade has potential advantages over femoral block by avoiding motor blockade of the quadriceps muscle and providing early ambulation [16].
Thigh tourniquets are commonly used during knee surgeries to reduce intraoperative blood loss and improve surgical outcomes [17-19]. Although a thigh tourniquet was shown to significantly increase the proximal–distal distribution of radiopaque dye within the adductor canal in a cadaver study, there are insufficient data regarding the occurrence of quadriceps weakness after proximal spreading of the local anesthetic agent [18]. We hypothesized whether performing ACB before or after inflation of a thigh tourniquet may also affect the spread of the local anesthetic agent, which may affect analgesia quality and quadriceps weakness. Thus, our study aimed to investigate the ideal timing for ACB and whether it should be performed before or after application of a thigh tourniquet.
Materials and Methods
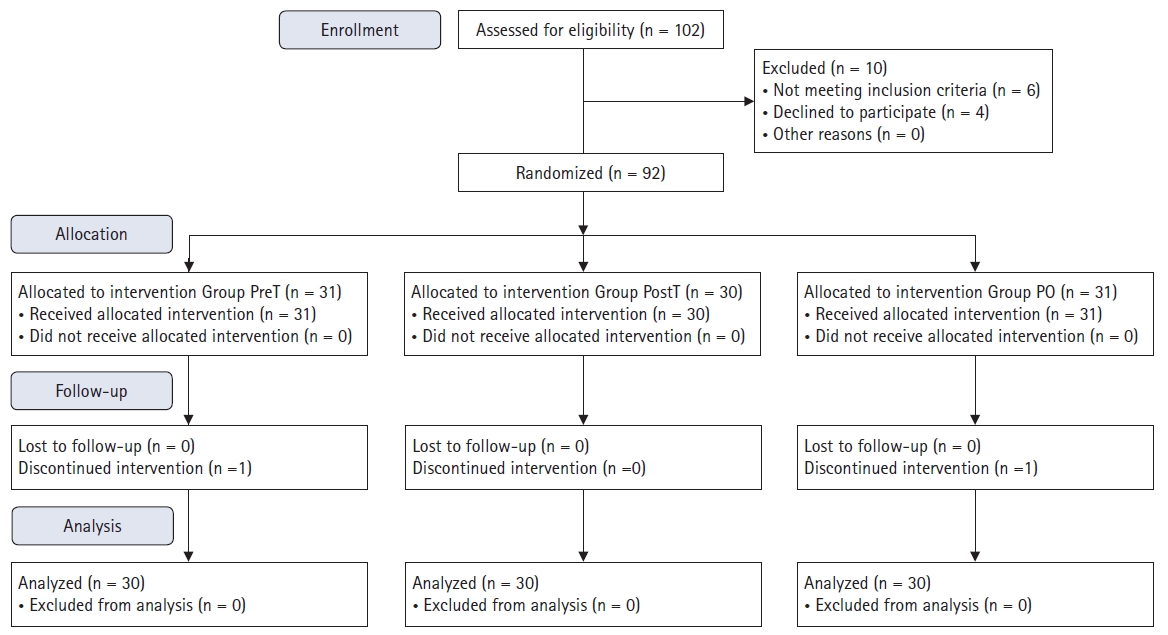
This randomized, prospective, exploratory study was approved by the ethics and research committee of Istanbul Medipol University (IRB number: 66291034-604.01.01-E.17567). After approval, the study was registered at ClinicalTrials.gov (registration number: NCT04010916). The Consolidated Standards of Reporting Trials (CONSORT) flow diagram was used for patient enrollment (Fig. 1). Written informed consent was obtained from all patients. The study was conducted between July 2019 and October 2020 at Medipol University Hospital. The study was conducted in accordance with the Helsinki Declaration-2013.
Ninety patients aged 18–65 years with American Society of Anesthesiologists (ASA) physical status classification I and II who were scheduled for unilateral arthroscopic knee surgery were enrolled in the study. Patients with a history of bleeding diathesis; patients who were pregnant or breastfeeding; patients with a history of anticoagulant treatment, allergy to local anesthetic or opioids, or infections at the site of block performance; and patients who refused block performance were excluded from the study. Using a computerized randomization program, the patients were equally divided into three groups (n = 30 in each group) according to the timing of ACB performance—the pre-tourniquet ACB group (PreT group), the after-tourniquet ACB group (PostT group), and the postoperative ACB group (PO group).
General anesthesia
After standard ASA monitoring in the operating room (electrocardiography, noninvasive blood pressure, and pulse oximetry [SpO2]) and premedication with 2 mg of intravenous (IV) midazolam, anesthesia induction was performed with IV propofol (2–2.5 mg/kg), fentanyl (1–1.5 µg/kg), and rocuronium bromide (0.6 mg/kg). Sevoflurane in a mixture of 50% air–oxygen with 2–3 L/min of fresh gas flow was used to maintain anesthesia. Analgesia was provided with a remifentanil infusion at a rate of 0.01–0.1 µg/kg/min during surgery. In cases of increased heart rate and mean arterial pressure above the baseline level, fentanyl (1 μg/kg) was administered. All personnel in the operating room were blinded to patient randomization. All surgical procedures were performed by the same surgical team using the same technique. At the end of surgery, the neuromuscular blockade was antagonized using IV atropine (0.01 mg/kg) and neostigmine (0.05 mg/kg). The patients were extubated after exhibiting sufficient spontaneous respiration and were transferred to the post-anesthesia care unit (PACU). After attaining a modified Aldrete score of ≥ 9, the patients were discharged from the PACU.
Adductor canal block procedure
After general anesthesia, all blocks were performed under ultrasound (US) guidance (Vivid q US device, GE Healthcare, USA) with a high-frequency (12 mHz) linear US probe and a 22 G, 50 mm block needle (Stimuplex Ultra 360; B. Braun, Germany). While ACB was performed before tourniquet inflation in the PreT group, it was performed after inflation of the tourniquet in the PostT group. In the PO group, ACB was performed at the end of surgery after deflation of the tourniquet. The participants were unaware of their group assignments.
• PreT group: ACB was performed preoperatively, before inflation of the tourniquet. The thigh tourniquet was inflated immediately after performing ACB.
• PostT group: ACB was performed preoperatively, after inflation of the tourniquet.
• PO group: ACB was performed postoperatively after deflation of the tourniquet.
The thigh tourniquet was inflated to 250–300 mmHg on the proximal aspect of the thigh using an electronic tourniquet system, supported with an Esmarch bandage, and was applied during surgery [16,17].
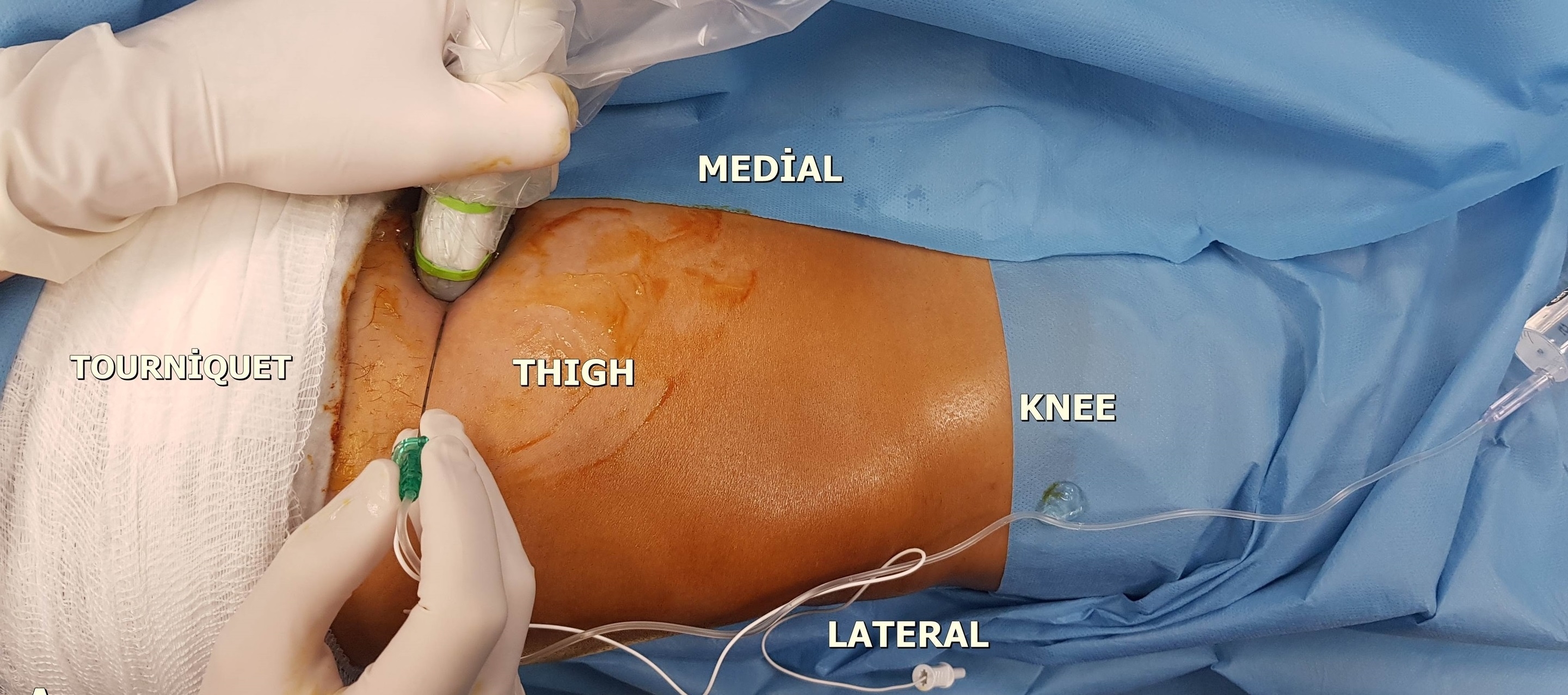
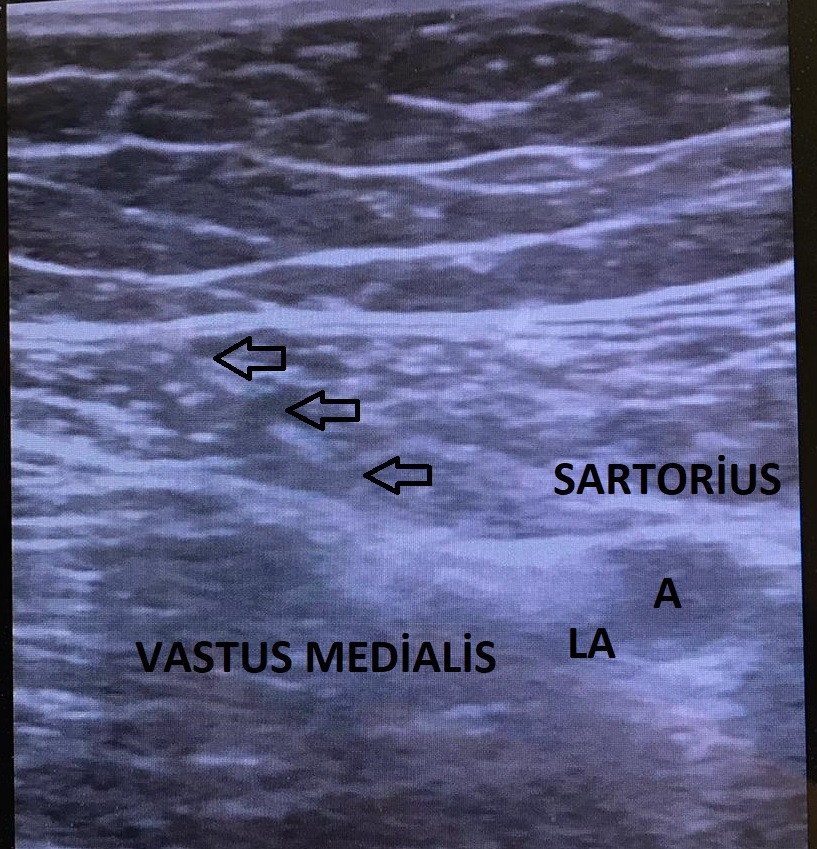
The US probe was placed at the mid-thigh, half the distance between the inguinal crease and the patella, and the adductor canal was identified (Fig. 2). After visualization of the pulsatile superficial femoral artery dorsal to the sartorius muscle, the probe was moved distally. At this level, the saphenous nerve was visualized as a hyperechoic structure lateral–anterior to the artery in the subsartorial region [4,5,11]. Using the in-plane technique, the injection site was confirmed with an injection of 5 ml of saline, and then 30 ml of 0.25% bupivacaine was injected (Fig. 3).
Outcomes and assessments—postoperative analgesia management, dermatomal testing, and motor block evaluation
The primary outcome was postoperative (24 h) opioid consumption, and the secondary outcomes were postoperative pain scores (visual analog scale [VAS] scores), quadriceps motor blockade, and adverse effects related to opioids (e.g., allergic reaction, nausea, and vomiting).
A standardized postoperative pain management protocol was used in this study. Twenty minutes before the end of surgery, IV ibuprofen 400 mg and IV tramadol 100 mg were administered. IV ibuprofen 400 mg was administered every 8 h in the postoperative period. A patient-controlled analgesia pump administering only fentanyl (10 µg/ml) was provided to all patients with a 2 ml bolus, no background infusion, a lockout time of 20 min, and a 4 h limit. Pain evaluation was performed using the VAS (0 = no pain, 10 = most severe pain). Static (at rest) and dynamic (during mobilization) VAS scores were recorded at 0 (PACU) and 2, 4, 8, 16, and 24 h postoperatively. If the VAS score was ≥ 4 with the routine analgesia protocol, only meperidine (0.5 mg/kg) IV was administered as a rescue analgesic. Postoperative opioid consumption was evaluated and recorded at 0–8, 8–16, and 16–24 h time intervals. Any opioid-related adverse effects, such as nausea, vomiting, or itching, were also recorded. The outcomes were evaluated and recorded by a single pain nurse anesthetist who was blinded to the study.
Dermatomal testing was performed using a pinprick sensation test 20 min after surgery along the field of the saphenous nerve (the medial infrapatellar region and the medial malleolus) by an anesthesiologist who did not participate in the study. The loss of sensation in the corresponding area is considered a successful block [4]. A single motor block evaluation was performed 20 min after surgery by an orthopedic surgeon who was blinded to the study. For motor block evaluation, the patient was asked to extend the knee from full flexion, and the block was classified as grade 0 (normal muscle power), grade I (motor weakness), or grade II (complete motor paralysis) [20].
Sample size calculation and statistical analyses
The primary aim of the study was to compare fentanyl consumption within 24 h postoperatively between the three groups. To determine the required sample size, a preliminary study was performed with 30 patients. While the mean fentanyl consumption was around 48 ± 16.8 µg in the PreT group (n = 10), it was 32 ± 13.9 µg in the PostT group (n = 10) and 36 ± 24.5 µg in the PO group (n = 10). For total opioid consumption, a sample size of 81 was calculated using G*Power (version 3.1.9.2, Germany) with an alpha probability of 0.05, a power of 0.95, and a medium-to-large effect size (0.4) [21]. Considering possible dropouts, we included 30 patients in each group to attain a higher power for a total of 90 patients.
Statistical analysis was performed using IBM SPSS ver. 20.0 (IBM SPSS Statistics Inc., USA) software package. The normality of variable distributions was assessed using the Kolmogorov–Smirnov test and histograms. Descriptive data are expressed as mean ± standard deviation (SD) or median [interquartile range (Q1, Q3)]. Categorical variables were analyzed using the Pearson’s chi-square test. Normally distributed data comprising continuous variables were analyzed using one-way analysis of variance, and non-normally distributed data comprising continuous variables were analyzed using the Kruskal–Wallis test to determine differences between groups. A P value of < 0.05 was considered statistically significant.
Results
Fig. 1 shows the CONSORT flow diagram, which describes the patients enrolled in the study. This randomized study included 90 patients, with 30 patients in each of the three groups (PreT, PostT, and PO groups). There were no statistical differences between the groups in terms of demographic data, anesthesia duration, or length of surgery (Table 1). ACB was successfully achieved in all patients.
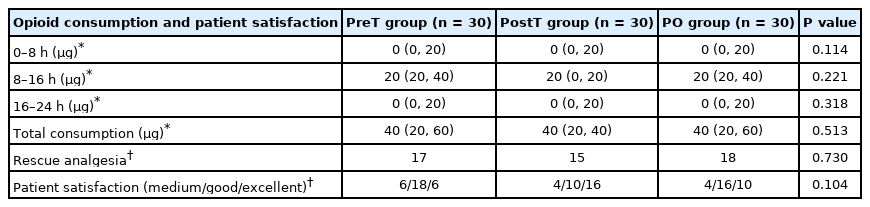
Opioid consumption, the primary outcome of the study, was not significantly different between the groups at any time interval (for total consumption; P = 0.513). The total consumption was 40 μg (20–60 μg) in the PreT group, 40 μg (20–40 μg) in the PostT group, and 40 μg (20–60 μg) in the PO group. The number of patients who received rescue analgesia (17 patients in the PreT group, 15 patients in the PostT group, and 18 patients in the PO group) and patient satisfaction were also not significantly different between the groups (Table 2). In addition, there was no significant difference in static and dynamic VAS scores between the groups (for 24 h; P = 0.306 and P = 0.271, respectively) (Table 3).
The incidence of motor block grade II was higher in the PreT group (eight patients) than in the PostT group (no patients) and the PO group (one patient; P = 0.005) (Table 4). The postoperative incidence of opioid-related side effects was also not significantly different between the groups (Table 4).
Discussion
The present study evaluated the efficacy of ACB before and after thigh tourniquet inflation in patients undergoing arthroscopic knee surgery. The results of this study showed no differences between groups in terms of either opioid consumption or pain scores. According to our results, applying a thigh tourniquet immediately after ACB contributes to the occurrence of motor blockade.
Local anesthetic agent distribution through the adductor canal is crucial because it may affect both analgesic efficacy and quadriceps weakness in ACB. The distribution of local anesthetic agents to distal locations (popliteal fossa) through the adductor canal may affect the analgesic efficacy of ACB after knee surgery [22]. The adductor canal extends to the apex of the femoral triangle; therefore, larger volumes of local anesthetic agents or continuous infusion may result in blockade of the femoral nerve [18,23]. The possible predictive factors in the distribution of local anesthetic agents include the injection location, the volume of local anesthetic agent, and whether the local anesthetic agent is given as a bolus or continuous infusion [22,24]. In a study investigating the distribution of the injectate and sensory-motor blockade, Gautier et al. [22] found that 20 ml of the local anesthetic resulted in spread into the popliteal fossa. In contrast, Andersen et al. [19] found that 15 ml of dye was sufficient to spread both proximally and distally through the adductor canal. Jaeger et al. [23] attempted to find the minimum effective volume (dose) of lidocaine 1% to fill the adductor canal and concluded that the minimum effective dose was 20 ml. According to Jaeger et al. [23], there was no correlation between the volume, proximal spread, and muscle strength. Anatomical differences and the fascia associated with the adductor canal may be predictors of the spread of local anesthetics. The similarity between these studies is that none involved the use of a tourniquet. However, the presence of a thigh tourniquet may be another factor that can affect local anesthetic or dye distribution. Nair et al. [18] investigated the effect of a thigh tourniquet on the distribution of local anesthetic within the adductor canal and found a combined superior–inferior dye distribution in cadavers. In the study, Nair et al. injected 25 ml of radio-opaque dye into the adductor canal and applied the tourniquet immediately after the ACB to simulate clinical practice. They found that tourniquets significantly increased the dye distribution proximally and distally. In the cadaveric study, Nair et al. concluded that the pressure created by the tourniquet may have increased the spread of the local anesthetic within the adductor canal.
As an explanation for quadriceps weakness in the PreT group, the pressure of the tourniquet inflated immediately after performing ACB may increase the spread of the local anesthetic within the adductor canal proximally and distally. Our results support the findings of the cadaveric study performed by Nair et al. [18]. This may be a result of the spread of local anesthetic to the motor fibers of the femoral nerve throughout the adductor canal.
This study has some limitations. First, we measured the motor block only once, 20 min after surgery. It would be optimal to determine the duration of motor weakness after surgery. Second, the assessment of motor weakness was subjective in nature. Future studies should utilize objective motor weakness testing. Lastly, because we tested motor function just 20 min after surgery, and the reversal of muscle relaxation was not confirmed, residual relaxation by intraoperatively administered muscle relaxants may have affected the outcome of motor function assessment.
In conclusion, using a tourniquet before or after ACB or performing ACB at the end of surgery after deflation of the tourniquet did not result in differences in terms of analgesia; however, applying a tourniquet immediately after ACB may lead to motor blockade. Further studies with lower volumes of analgesics are required to confirm our findings.
Notes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Mursel Ekinci (Conceptualization; Investigation; Methodology; Visualization; Writing – original draft)
Bahadir Ciftci (Conceptualization; Data curation; Investigation; Methodology; Supervision; Visualization; Writing – original draft)
Yavuz Demiraran (Conceptualization; Methodology; Visualization; Writing – review & editing)
Erkan Cem Celik (Conceptualization; Data curation; Formal analysis; Investigation; Software)
Murat Yayik (Conceptualization; Data curation; Investigation; Methodology)
Burak Omur (Conceptualization; Investigation; Methodology)
Ersin Kuyucu (Conceptualization; Investigation; Methodology)
Yunus Oktay Atalay (Conceptualization; Data curation; Investigation; Methodology; Writing – review & editing)