Comparative study of levobupivacaine and bupivacaine for bilateral maxillary nerve block during pediatric primary cleft palate surgery: a randomized double-blind controlled study
Article information
Abstract
Background
Cleft lip and palate are common major congenital anomalies. Cleft palate (CP) repair causes pain and needs large doses of intravenous opioids. The risk of postoperative airway obstruction or respiratory depression is high, requiring continuous and vigilant monitoring. The primary outcome was to evaluate the efficacy of using different local anesthetics during bilateral maxillary nerve block (MNB) with general anesthesia on quality of recovery after primary CP repair. We hypothesized that levobupivacaine would be better than bupivacaine. Also, to investigate the potency of bilateral MNB in improving quality of postoperative analgesia.
Methods
Sixty children undergoing primary CP repair surgery were enrolled in the study. Combined general anesthesia and regional bilateral MNB were used for all patients. Group L (n = 30): children received 0.15 ml/kg of 0.2% levobupivacaine, while in Group B (n = 30): children received 0.15 ml/kg of 0.2% bupivacaine.
Results
Face, Legs, Activity, Cry, and Consolability pain score readings were 0 score in 7 cases of the Group L and 10 cases of Group B, 1 score in 14 cases of the Group L and 12 cases of Group B, and 2 score in 9 cases of the Group L and 8 cases of Group B. We found no statistically significant difference between the two study groups as regarding pain score or serious complications.
Conclusions
Levobupivacaine is as effective and safe as bupivacaine to be used for MNB block with a lower incidence of complications. Bilateral suprazygomatic MNB is an effective, easy, and safe method for pain relief in children undergoing primary cleft palate repair surgeries.
Introduction
Cleft lip and palate is a very common major congenital abnormality [1]. Primary surgery for cleft palate (CP) can vary depending on the surgical procedures used, the age of the patient at operation, the anesthetic technique used, and the postoperative management course [2]. CP repair causes pain and necessitates large doses of intravenous (IV) opioids [3,4]. The risk of postoperative airway obstruction and respiratory depression is high and requires vigilant monitoring, particularly during the first 24 hours postoperatively [5].
Adequate postoperative pain relief is a critical concern in the perioperative care of children. Good analgesia minimizes oxygen requirements, decreases cardio-respiratory demands, and allows for early ambulation and discharge. Regional nerve block provides good preemptive analgesia when given in combination with general anesthesia (GA). It is associated with hemodynamic stability, rapid recovery, and reduced supplemental analgesia during the postoperative period [4].
Maxillary nerve block (MNB) is technically easy to perform. Early work has suggested few complications, improved analgesia, decreased perioperative opioid consumption, and the possibility of early feeding after surgical repair of CP [6]. Bilateral MNB is done by a suprazygomatic approach [7] and may be guided by findings from computed tomography [6]. Innervation of the soft and hard palates is from the greater and lesser palatine nerves, which both pass through the sphenopalatine ganglion [8]. Sensory innervation of the anterior and posterior palate is from the maxillary nerve (MN) [9].
The primary aim of this study was to determine the efficacy and safety of different local anesthetics, used during bilateral MNB in conjunction with GA, with respect to reducing the requirement for supplemental analgesia during the postoperative course following primary CP repair. We hypothesized that the local anesthetic levobupivacaine would be better than bupivacaine. A secondary aim was to investigate the potential of bilateral MNB to improve the quality of postoperative pain relief and incidence of adverse effects.
Materials and Methods
Eligibility and randomization
After approval from our Faculty Ethical Committee (ref. no. IRB00008718), the parents of all participants were provided with information about the anesthesia and analgesia techniques that would be applied to their children. Written informed consent was obtained from each parent. Clinical trials registration was approved under NCT02923869, and the study began in January 2016 and finished in October 2016.
Study design
A prospective randomized controlled double-blind study using a computer-generated randomization scheme was carried out at Assiut University Hospital on 60 children undergoing primary CP repair surgery. Combined GA with regional bilateral MNB was used for anesthesia in these surgeries. The study anesthetics used in the MNB were prepared by a second anesthetist to maintain blinding. The MNB and study parameters (intraoperative and postoperative) were performed and recorded, respectively, by the first anesthetist.
Sample size
The sample size was calculated to allow for detection of a 50% reduction of rescue analgesia (nalbuphine) for each group in the 6 hours post-surgery. With a power of 90% and type I error of 5%, 30 patients were required in each group (α = 0.05 and β = 90%). Children were randomly allocated into one of two groups, each comprising 30 patients. Group L children received 0.15 ml/kg of Levobupivacaine 0.2% on each side (maximum 4 ml) and Group B children received 0.15 ml/kg of bupivacaine 0.2% on each side (maximum 4 ml).
Inclusion criteria
Children 1–10 years of age, of either sex, who were American Society of Anesthesiologists I or II and had a primary CP.
Exclusion criteria
Children who had an allergy to local anesthetics, any coagulation disorder, local infection or injury at the site of MNB, concomitant rhinoplasty, any additional congenital anomalies, a history of upper or lower airway disease, or of sleep apnea, were excluded from the study.
Preoperative assessment
The day before surgery, all children participating in this study had undergone pre-anesthetic workup to assess preoperative fitness, including a detailed history (from the parents) and a thorough physical examination. Weight and height of all children were carefully recorded.
Monitoring
Electrocardiography (ECG), pulse oximetry (SpO2), non-invasive mean arterial blood pressure (MAP), end-tidal CO2 (ETCO2), and non-invasive temperature probes were used to monitor each patient.
Anesthetic technique
Premedication: All children received intramuscular midazolam 0.05 mg/kg 10–20 minutes before induction of GA.
Following 3 minutes of pre-oxygenation with 100% O2, GA was induced by sevoflurane inhalation 4–6% via a facemask. An IV line was secured and IV 0.9% NaCl was started at the calculated volume and rate. Fentanyl 1 μg/kg and propofol 1.5 mg/kg were given intravenously. Intubation was performed with an oral RAE tube of the appropriate size. Assisted ventilation was adjusted to maintain an ETCO2 of 30–35 mmHg. Anesthesia was maintained with 100% O2 with 2–4% sevoflurane. A broad-spectrum antibiotic was given to each child participating in the study.
Suprazygomatic MNB
Bilateral suprazygomatic MNB was performed after complete aseptic preparation of the skin, but prior to incision, in the anesthetized patient. The patient was maintained in a supine position with the head in a neutral position. The puncture site chosen was at the fronto-zygomatic angle, at the junction of the upper edge of the zygomatic arch and the frontal bone. A 25-G needle, 50 mm in length, was inserted perpendicular to the skin and redirected toward the nasolabial fold. The pterygopalatine fossa was the target at approximately 35–45 mm from the puncture site of the skin. Negative blood aspiration was performed prior to injecting the study solution (levobupivacaine or bupivacaine) on each side. The success of MNB was indexed by a lack of any sympathetic response to surgical stimulation.
Data collection
Demographic data were collected including age, sex, weight, and height.
Heart rate (HR), MAP, ECG, SpO2, ETCO2, and sevoflurane % were measured every 15 minutes after MNB until the end of the surgery.
Postoperative HR, MAP, SpO2, and respiratory rate (RR) were measured at the time of pain assessment.
Pain severity was assessed by the first anesthetist (who was blinded to the study drug) on arrival at the recovery room, and at 1, 2, 4, 6, 8, 12, and 24 hours according to the Face, Legs, Activity, Cry, and Consolability (FLACC) pain score [10]. If the FLACC score was ≥ 3, IV nalbuphine 0.1 mg/kg was given for supplemental analgesia.
The time at which the first analgesic was administered, the total dose of nalbuphine consumption, and any adverse effects (including sedation, vomiting, respiratory depression, hypoxia, bleeding at the puncture site, or any systemic toxicity related to the local anesthetic) were also recorded.
The sedation score was on by a four-point scale (1–4), as follows: 1) Awake and alert, 2) Sedated and responding to verbal command, 3) Sedated and responding to mild stimulus, and 4) Sedated and responding to moderate to severe physical stimulus [11].
Blood glucose levels were measured before MNB, 15 minutes after nerve block, and 2 hours after the end of surgery.
Statistical analysis
All data were collected and processed using SPSS software (ver. 20.0; SPSS Inc., USA). All results were expressed as mean (SD) and ranges (as numbers or percentages). Categorical data were compared using a chi-square test. Non-parametric data were compared using the Mann-Whitney U test. Numerical data were compared using the independent samples Student’s t test. P < 0.05 was considered significant.
Results
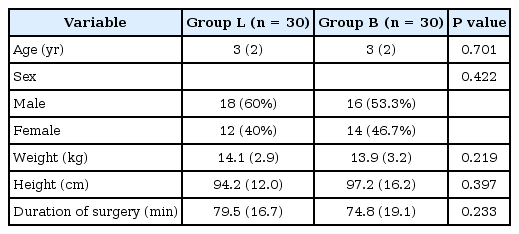
All children included in this randomized, double-blind study were discharged from the hospital within 36 hours of surgery. The suprazygomatic nerve block was successful in all patients and no cases were excluded from the analysis. There was no statistically significant difference in demographic data or clinical characteristics (age, sex, weight, height, and duration of operation) between the two study groups (Table 1).
There was no statistically significant difference between the two groups, during the intraoperative and postoperative periods, in MAP, oxygen saturation, or ETCO2. With respect to HR, there was a slight decrease in the intraoperative versus preoperative (baseline) readings in both groups, with no statistically significant difference between the two groups.
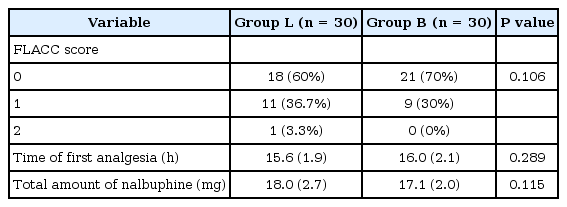
Children in both groups reported a low FLACC pain score throughout the postoperative follow-up period, with no statistically significant difference between the two study groups (Table 2).
The mean time at which participants required their first dose of the nalbuphine supplemental analgesia was 15.6 (1.9) hours in group L and 16.0 (2.1) hours in group B, with no statistically significant difference between the two groups. A total of 18.0 (2.8) mg of supplemental analgesia was required in group L, versus 17.1 (2.1) mg in group B, with no statistically significant difference between the two groups (Table 2).
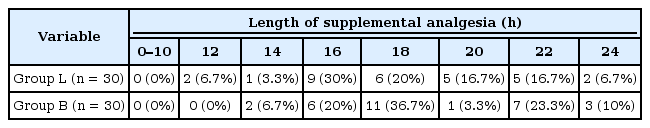
The median time at which supplemental analgesia (nalbuphine) was given in group L was 12 hours postoperatively, with a median of 15 hours (range: 12–24 hours); in group B, nalbuphine was given after a median time of 14 hours postoperatively with a median of 17 hours (range: 14–24 hours) (Table 3).
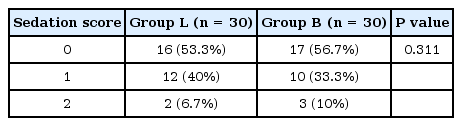
Concerning sedation scores, no significantly prolonged sedation was recorded in either study group and there was no statistically significant difference between the groups throughout the postoperative follow-up period (Table 4).
A similar sevoflurane % concentration was used throughout the procedure in both groups, with no statistically significant difference (Table 5). Serum glucose levels were also recorded preoperatively, and at 15 minutes and 2 hours post incision; there was no statistically significant difference between the two study groups (Table 5).
All children reported few complications, either intraoperatively or within the first 24 hours postoperatively. Two instances of vomiting, five cases of local edema at the site of surgery, and two cases of bleeding at the puncture site of nerve block, which stopped once external compression was applied, were reported throughout the study. No cases of dysphagia, mouth opening limitations, or other serious complications were noted.
Discussion
Prophylactic analgesia using a local nerve block is being increasingly used in generally anesthetized young children and has demonstrated a good safety profile. Peripheral nerve block provides specific analgesia at the surgical site and has a prolonged duration of action (depending on the medications chosen) [12]. Although regional anesthesia has a good safety profile, the global experience with pediatric regional anesthesia is still low [13].
Regional anesthesia usually decreases the intraoperative requirement for anesthetics (inhaled or IV) and allows for rapid recovery while providing effective postoperative analgesia with minimal sedation [14]. We noted that no participants required an increase in their sevoflurane concentration above the established 2–3% threshold; this suggests that local anesthesia may lead to a decrease in the requirement for intraoperative sevoflurane concentration.
Regional anesthesia with local anesthetics has been shown to inhibit the surgical stress response and can influence the postoperative outcome due to its positive effects on organ function [15]. In contrast to opioids, local anesthetics can be used safely and, according to recent guidelines, regional anesthesia is now accepted as the cornerstone of postoperative analgesia in pediatric patients [16].
Levobupivacaine is preferred in clinical practice because of its safe pharmacological profile. Regarding regional anesthesia, the incidence of cardiac and neurological complications was shown to be significantly higher with bupivacaine compared to levobupivacaine [17]. This led us to perform a comparative study of levobupivacaine and bupivacaine, with the ultimate aim of removing the need for bupivacaine in MNBs done during CP repair surgery. The overall incidence of neurological injury, cardiac, and central nervous system toxicity after peripheral nerve block is lower when bupivacaine is used. Higher concentrations of levobupivacaine are required to produce cardiac and neurotoxicity compared to a racemic mixture of bupivacaine [18].
We also noted no serious intraoperative or postoperative complications in either group in this study, indicating the safety of these doses of levobupivacaine and bupivacaine for nerve block in children during surgery.
Many studies have compared the criteria for selecting bupivacaine, levobupivacaine, and ropivacaine for peripheral nerve block. Equal doses of bupivacaine 0.5% and levobupivacaine can result in sensory and motor blocks of similar onset (6–10 minutes) and duration (14–16 hours) [19]. We have demonstrated the long-lasting effects of postoperative analgesia after nerve block, with a median duration of action of 12 hours with levobupivacaine and 14 hours with bupivacaine.
CP surgery is very painful, especially during the 24–48-hour period following surgery. Analgesic management varies widely among different surgical teams. Local nerve blocks improved anesthetic care management, as well as the perioperative and postoperative follow-up [20]. In another study, the suprazygomatic approach was preferred since it has been shown to lead to a lower complication rate versus other approaches [7]. This simple, easy, reliable, and virtually risk-free technique can provide an effective and prolonged analgesia, with a decreased requirement for morphine analgesics during and after cleft lip-palate repair surgery in young children [6]. These findings are in line with our work. We performed bilateral suprazygomatic maxillary nerve block (SMB) in 60 cases and achieved a 100% success rate, demonstrating that this technique can be performed with ease.
These results were the same as those of Jonnavithula et al. [20], who showed that the efficacy of greater palatine nerve block in the management of postoperative analgesia led to greater parent satisfaction with CP surgery. They reported significantly lower pain scores in the nerve block group, a decreased need for supplemental analgesia, and better parental satisfaction for the nerve block versus GA group [20].
Sathitkarnmanee et al. [21] compared 3 ml isobaric bupivacaine 0.5% with levobupivacaine and showed that there was no significant difference between them regarding the criteria for sensory and motor blocks. A similar study demonstrated concordant results with regard to the use of SMB for pain relief. SMB allowed for lower opioid consumption during the first 48 hours postoperatively [5]. Our results are in agreement with these findings; we found better postoperative analgesic effects with SMB. We showed that SMB allowed for lower overall 24-hour nalbuphine consumption [18.04 (2.75) mg/day in the group L and 17.11 (2.08) in group B].
Another study compared systemic pethidine with regional nerve block in children during CP repair surgery. There was a lower requirement for postoperative rescue analgesia in patients with regional blocks [22]. Some investigators reported lower pain scores during the first 2 postoperative hours, and a lower requirement for supplemental analgesia in children after palatal infiltration with lidocaine [23].
We believe that the addition of regional nerve blocks to general anesthetics would afford better glycemic control in children. This is in line with the conclusion arrived at in a study showing no significant difference between GA alone versus regional anesthesia and GA in a pediatric population, with regard to the metabolic response [24].
Limitations
First, we did not include a GA control group, receiving sevoflurane alone, to assess whether adding any regional local anesthetic to GA would result in similar findings. However, we did perform this comparison in a previous study.
Second, real-time ultrasonography could not be used in conjunction with SMB to decrease the incidence of complications, although we recorded no serious complications due to the technique or drugs used.
Third, a larger number of patients may be required to evaluate the blind technique used, and to detect any possible complications.
Finally, we had difficulty in evaluating the success rate of the block because it was done in generally anesthetized patients. Therefore, we could not report the exact failure rate.
Conclusion and recommendations
Levobupivacaine is as effective and safe as bupivacaine when used for MNB block, but with a lower incidence of complications. Bilateral SMB is an effective, easy, and safe method for pain relief in children undergoing primary CP repair surgery, with no serious complications.
We recommend that SMB be performed with the aid of ultrasound to increase the success rate in inexperienced anesthesiologists, especially for procedures performed in young children.
Acknowledgements
With special thanks to “Operation Smile Egypt” organization, affiliated to “Operation Smile International, USA” helped a lot to build up this work, we are volunteers with this organization many years ago.