Neuroprotective effect of erythropoietin on anesthesia-induced neurotoxicity through the modulation of autophagy in Caenorhabditis elegans
Article information
Abstract
Background
The anti-oxidative, anti-inflammatory, and anti-apoptotic effects of erythropoietin may provide neuroprotective effects. Erythropoietin also modulates autophagy signaling that may play a role in anesthesia-induced neurotoxicity (AIN). Herein, we investigated whether AIN can be attenuated by the neuroprotective effect of erythropoietin in the Caenorhabditis elegans (C. elegans).
Methods
Synchronized worms were divided into the control, Iso, EPO, and EPO-Iso groups. The chemotaxis index (CI) was evaluated when they reached the young adult stage. The lgg-1::GFP-positive puncta per seam cell were used to determine the autophagic events. The erythropoietin-mediated pathway of autophagy was determined by measuring the genetic expression level of let-363, bec-1, atg-7, atg-5, and lgg-3.
Results
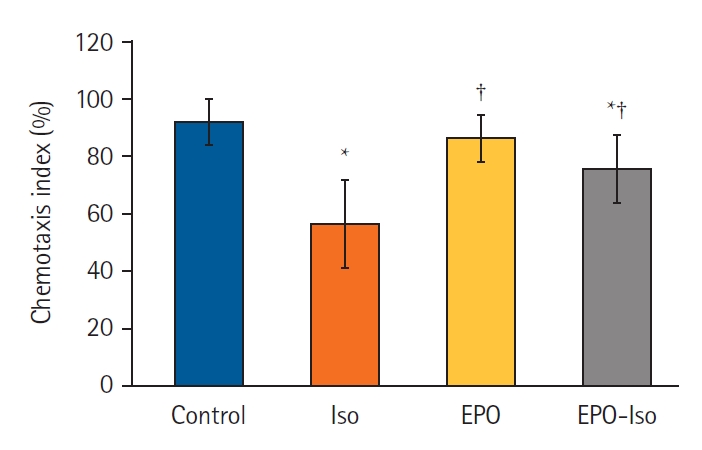
Increased lgg-1::GFP puncta were observed in the Iso, EPO, and EPO-Iso groups. In the Iso group, only the let-363 level decreased significantly as compared to that in the control group (P = 0.009). bec-1 (P < 0.001), atg-5 (P = 0.012), and lgg-3 (P < 0.001) were expressed significantly more in the EPO-Iso group than in the Iso groups. Repeated isoflurane exposure during development decreased the CI. Erythropoietin could restore the decreased CI by isoflurane significantly in the EPO-Iso group.
Conclusions
Erythropoietin showed neuroprotective effects against AIN and modulated the autophagic pathway in C. elegans. This experimental evidence of erythropoietin-related neuroprotection against AIN may be correlated with the induced autophagic degradation process that was sufficient for handling enhanced autophagy induction in erythropoietin-treated worms.
Introduction
Volatile anesthetics can cause apoptosis or degeneration of nervous cells in the developing brain in several experimental animal models [1–3]. This is known as anesthesia-induced neurotoxicity (AIN). Several mechanisms such as rapid increase in oxidative stress [4] and reactive oxygen species [5–7] are known to be two of the causes of AIN. Additionally, the potential role of autophagy is proposed in the AIN [8], and the modulation of autophagy may minimize the AIN [9,10]. Autophagy is a lysosome-mediated pathway for the degradation of misfolded proteins [11]. Recent studies showed the accumulation of autophagosomes and increased expression of autophagy-related markers in various pathologic conditions [12–14].
Erythropoietin is a representative hematopoietic cytokine. In the field of neuroscience, erythropoietin is being studied for its neuroprotective function. Erythropoietin is known to inhibit glutamate cytotoxic action, increase the expression of antioxidant enzymes, suppress the production of free radicals, and affect the secretion of neurotransmitters [15–17]. The anti-oxidative, anti-inflammatory, and anti-apoptotic effects of erythropoietin can result in neuroprotection, and it also modulates autophagy signaling [18,19].
Caenorhabditis elegans (C. elegans) does not have a vascular system; therefore, the hematopoietic effect of erythropoietin can be excluded from this model and the neuroprotective effect against AIN can be identified. Additionally, C. elegans is a proper animal model for studying autophagy due to the characterized autophagic genes and reliable visual monitoring of autophagy. [20].
Herein, we investigated whether AIN can be attenuated by the neuroprotective effect of erythropoietin. Accordingly, we aimed to identify the step of the genetic pathways related to autophagy that is affected by AIN and protected by erythropoietin in C. elegans.
Materials and Methods
Worm strains, culture, synchronization, and pharmacologic application
Wild-type N2 and adIs2122 [lgg-1p::GFP::lgg-1 + rol-6(su1006)] that were obtained from the Caenorhabditis Genetics Center (Minneapolis, MN, USA) were used. Escherichia coli strain OP50 was seeded in the nutrient growth medium (NGM) petri plates (OP50 plates) and all the worms were maintained on OP50 plates at 20°C. Egg-laying adult worms were incubated for 1 h and removed. Newly laid eggs hatched after 12 h; thus, synchronized L1 larvae were obtained for the next experiments.
Synchronized worms were divided into the following four subgroups: the control, Iso, EPO, or EPO-Iso group. All worms of the Iso and EPO-Iso groups were exposed to isoflurane four times, for 1 h at each larval stage. C. elegans was anesthetized as described previously [21,22]. Briefly, petri plates containing C. elegans were placed in a sealed glass chamber. Thereafter, liquid isoflurane was administered through a port mounted on the glass chamber; the isoflurane vaporized inside, anesthetizing the exposed worms. The isoflurane concentration was 99% effective dose for the worms. The worms of the EPO and EPO-Iso groups were treated with erythropoietin during the development period. Erythropoietin of the designated volume and concentration was spread with OP50 in the NGM plate. The erythropoietin-containing OP50 plates were replaced every 12 h. Erythropoietin concentrations of 3, 10, 30, 100, and 300 IU/ml were tested in each OP50 plate to determine the appropriate neuroprotective erythropoietin concentration (Fig. S1), and 5 IU/ml of erythropoietin was used in the subsequent experiments.
Chemotaxis assay
Nine centimeter NGM petri plates were prepared, with the starting (S) point marked at the center, attractant (A) point marked on one side, and control (C) point marked on the other side (Fig. 1). Then, 30 µl of OP50 suspension was seeded at point A for the attractant, while points S and C were left unseeded. When approximately 50 worms reached the young adult stage, they were moved to the point S of the chemotaxis petri plate after being washed three times with S-basal buffer. The worms in the chemotaxis plate were allowed to move for 1 h, and the number of worms in each section was counted under microscopy. The chemotaxis index (CI) was calculated using the following equation: (worm count of point A – worm count of point C) / total count of worms × 100 (%).
Analysis of autophagic events
A synchronized adIs2122 strain expressing lgg-1::GFP was used for the autophagy analysis. They were exposed to isoflurane four times as described above and cultivated on erythropoietin-containing NGM petri plates or not according to the group arrangement. When they reached the young adult stage, the transgenic worms were mounted on a 2% agarose pad in M9 buffer containing 0.1% NaN3 onto microscope slides and imaged using a fluorescence confocal microscope. Autophagic events were observed by evaluating the lgg-1::GFP-positive puncta per seam cell. Five independent experiments were performed and 10 worms were evaluated in each experiment. At least 5–10 seam cells from each worm were examined.
RNA preparation and real-time polymerase chain reaction (PCR)
The erythropoietin-mediated pathway of autophagy was determined by measuring the genetic expression levels of let-363, bec-1, and atg-7 using real-time PCR. RNA was extracted from young adult worms in each group with TRIzolTM reagent (Invitrogen, MA, USA). The quality-controlled RNA (OD 260/280 > 1.5 and OD 260/230 > 1.0) was confirmed by a NanoDropTM (ND-2000, Thermo Fisher Scientific, MA, USA) and an Agilent 2100 BioanalyzerTM (Agilent Technologies, Palo Alto, USA). The first-strand cDNA was synthesized by Maxima H minus First-strand cDNA Synthesis KitTM (Thermo Fisher Scientific, MA, USA), and each of the gene-specific primers was as follows: let-363, 5ʹ-AAT TAT AGA TTA CCC TGT TCC ACC A-3ʹ (forward) and 5ʹ-TGG AGA ATC GTT GCT CAA TGG-3ʹ (reverse); bec-1, 5ʹ-CTG TCA GCA TCC GTT GAG GT-3ʹ (forward) and 5ʹ-TGA GCA TCC GAG ATG AGC TTC-3ʹ (reverse); atg-7, 5ʹ-CGC TAT CGC TGC ACA GTT TC-3ʹ (forward) and 5ʹ-CCC GGT GAC TTC TTC GAG AC-3ʹ (reverse); atg-5, 5ʹ-TCG GCA CAG TTG TGG AGA AG-3ʹ (forward) and 5ʹ-ACC CGT AGT GGA ATG TGT GC-3ʹ (reverse); lgg-3, 5ʹ-TCC AAC GGA CAC TGT TGC TT-3ʹ (forward) and 5ʹ-GTG TCA GGA GAT GGG GCA AA-3ʹ (reverse); and pan-actin, 5ʹ-TCG GTA TGG GAC AGA AGG AC-3ʹ (forward) and 5ʹ-CAT CCC AGT TGG TGA CGA TA-3ʹ (reverse).
Statistics
Data are presented as the mean ± standard deviation; they were tested for normality using the Shapiro-Wilk test. Normally distributed data were analyzed using a one-way analysis of variance; for post-hoc analysis, Bonferroni or Games-Howell correction was used according to equal or unequal variances, respectively. Statistical analyses were performed using the SPSS® 27.0 version (IBM Co., Armonk, NY, USA) and P values of 0.05 were considered statistically significant.
Results
The autophagic marker, lgg-1::GFP, was observed in young adult worms. The control group displayed little localization of lgg-1::GFP (Fig. 2A). An increased lgg-1::GFP expression was observed in the Iso and EPO groups (Fig. 2B and 2C), suggesting that autophagy in C. elegans was enhanced by either repeated isoflurane exposure or erythropoietin treatment. When C. elegans was pretreated with erythropoietin, the increase of lgg-1::GFP continued even after isoflurane exposure (Fig. 2D). The Iso, EPO, and EPO-Iso groups presented significantly higher numbers of lgg-1::GFP puncta per hypodermal seam cell than did the control group (Fig. 2E).
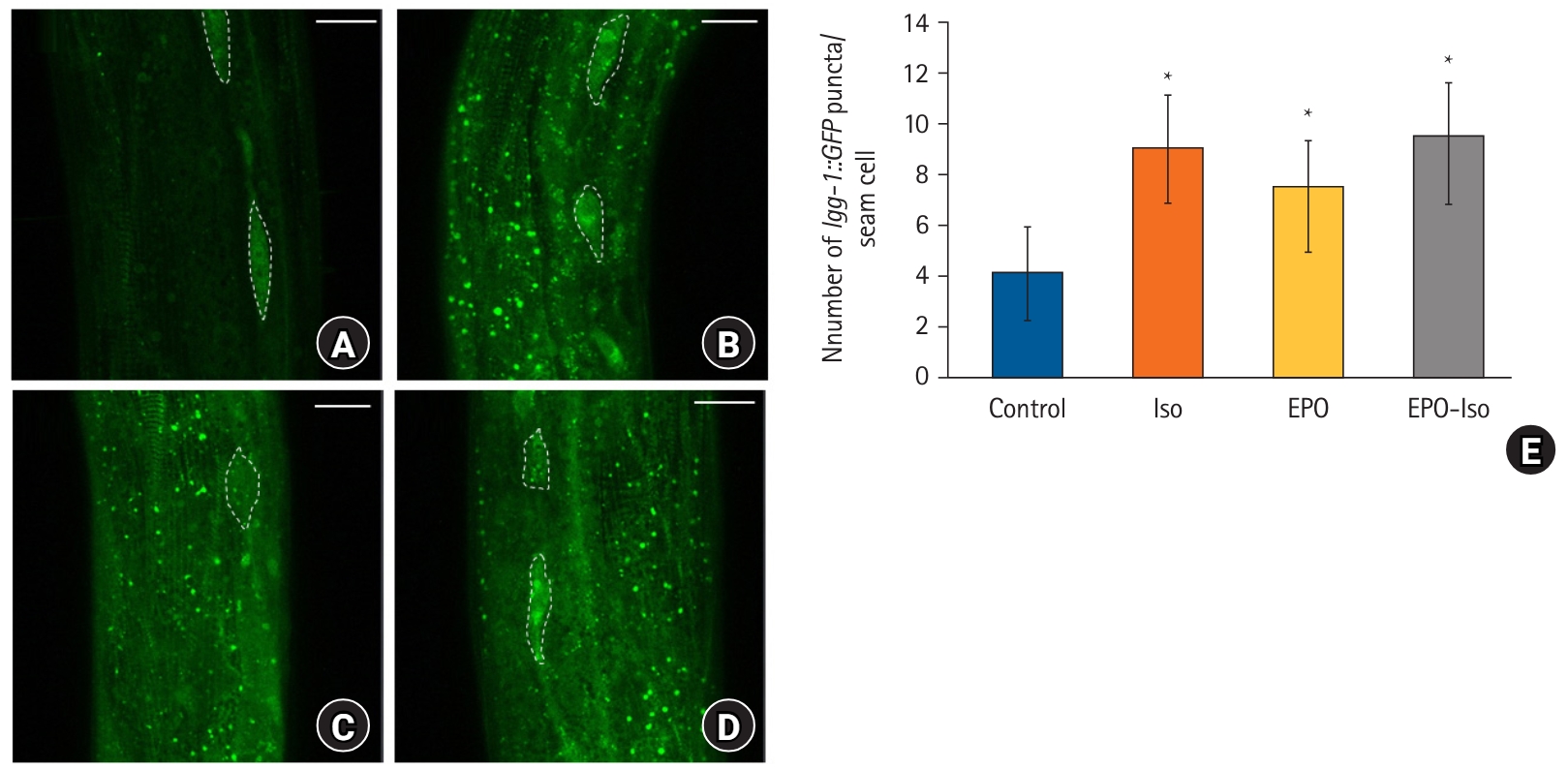
The expression pattern of lgg-1::GFP. (A) Control group; (B) Iso group; (C) EPO group; (D) EPO-Iso group; (E) number of lgg-1::GFP puncta per seam cell. Five independent experiments were performed and 10 worms were evaluated in each experiment. White dashed lines indicate seam cells; 5–10 seam cells were screened for each worm. The scale bar is 20 µm. *P < 0.05 compared to the control group. Other intergroup comparisons revealed no significant differences.
The up-regulation of autophagy can be either beneficial or harmful to organelles. To discriminate between these two roles, the expression of several autophagy-related genes was evaluated, such as let-363, bec-1, atg-7, atg-5, and lgg-3 in C. elegans (Fig. 3). The let-363 expression was significantly lower in the Iso group than in the control group (P = 0.009); however, the bec-1, atg-7, atg-5, and lgg-3 expressions did not differ significantly between the control and Iso groups. The let-363 expression in the EPO-Iso group was significantly lower than that in the control group (P < 0.001); however, it did not differ significantly from that in the ISO group (P = 0.176). bec-1 (P < 0.001), atg-5 (P = 0.012), and lgg-3 (P < 0.001) were expressed significantly more in the EPO-Iso group than in the Iso groups.
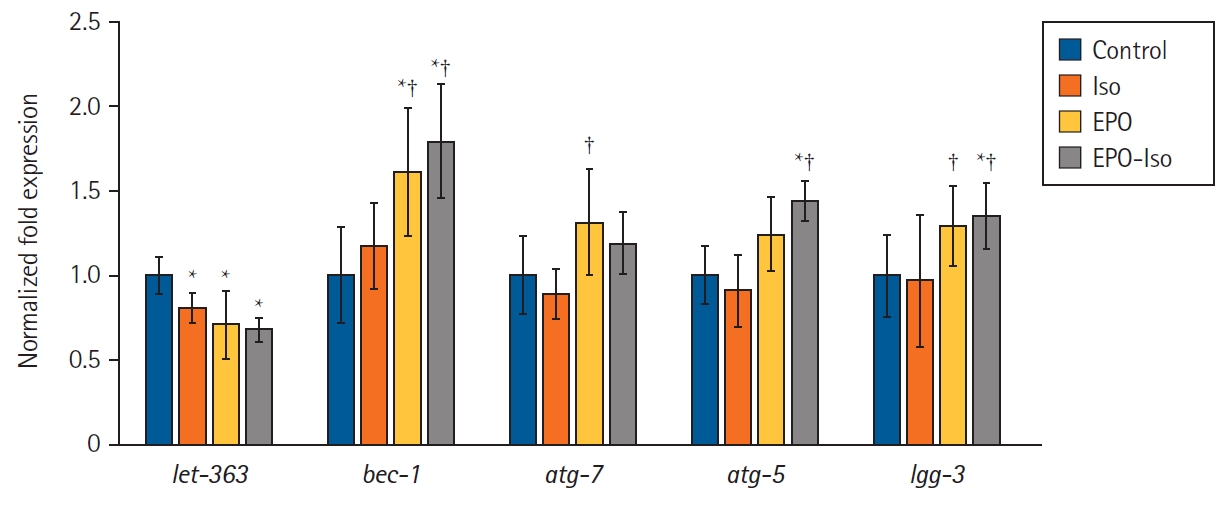
The expression level of autophagy-related genes. Graphs shown are averages with the standard deviation from five independent experiments including at least six petri plates per group in each experiment. Each petri plate contained at least 50 worms. *P < 0.05 compared to the control group; †P < 0.05 compared to the Iso group.
Repeated isoflurane exposure during the developmental period decreased the CI significantly (P < 0.001; Fig. 4) as shown in our previous studies [23,24]. Erythropoietin treatment without isoflurane exposure did not affect the CI. Erythropoietin in the EPO-Iso group could restore the decreased CI by isoflurane significantly (P < 0.001 for ISO vs. EPO-Iso); however, it did not recover the CI to the level in the control group (P = 0.018 for control vs. EPO-Iso).
Discussion
In the present study, repeated isoflurane exposure induced an increase in autophagosome formation without altering the autophagic degradation process in C. elegans. Neurotoxic effects were determined on the basis of the decrease in the CI in the isoflurane-treated worms. Erythropoietin pretreatment may reduce the neurotoxic effects of repeated isoflurane exposure by inducing autophagic degradation.
Autophagy is a fundamental degradation process to maintain homeostasis by eliminating misfolded proteins or damaged organelles. Considering that the extinction of autophagy causes axonal degradation and neural death in animal models [25,26], this autophagy process is also critical in neuronal homeostasis [27]. Autophagy is also induced by various stressful conditions, such as nutritional deprivation, hypoxia, endoplasmic reticulum stress, and pathogens [28]. Inhalation anesthesia was proposed to induce autophagy that interestingly plays a dual role, cytotoxic or cytoprotective [9,29]. The role of autophagy in the AIN has been evaluated in previous studies that reported that cognitive impairment or neurotoxicity by sevoflurane was related to excessive autophagy in the brains of young mice and fetal rats [10,30]. Prolonged anesthetic exposure can cause excessive autophagy and apoptosis that ultimately determines neural cell injury by anesthetic agents [31]. In our model, induced autophagy was also presented by repeated anesthesia exposure with decreased CI.
Erythropoietin fundamentally stimulates red blood cells in the bone marrow; however, its role in neuroprotection and neurogenesis has been studied in various neurodegenerative diseases, such as Alzheimer’s [32,33] and Parkinson’s diseases [34,35]. Autophagy is reported as a major neuroprotective mechanism of erythropoietin [36]. Jang et al. demonstrated the erythropoietin-induced neuroprotection showing the experimental evidence that the autophagy-related signaling pathway was enhanced by erythropoietin in the rotenone-induced neurotoxicity model [19]. Additionally, erythropoietin is reported to generate a protective effect by modifying the autophagic activity in oxygen-induced neurotoxicity [37]. Besides the neuroprotective effect of erythropoietin, it activated autophagy in other conditions, such as inflammatory microenvironment [38], spinal cord injury [18], or hepatic steatosis [39].
The neuroprotective effect of erythropoietin in AIN has been reported previously. Erythropoietin treatment attenuated sevoflurane-induced neuronal cell apoptosis in rat cortical neurons, where the other mechanism, the EPOR-Erk1/2-Nrf2/Bach1 signal pathway, was proposed [40]. Single treatment of erythropoietin could reduce both apoptosis and late neurologic disorders in newborn rats exposed to sevoflurane [41]. Autophagy-related neuroprotection by erythropoietin in AIN was first observed in the present study.
The gene lgg-1, an ortholog of LC3 in mammals, is observed in the seam cells, intestinal cells, or hypodermis of C. elegans, and is used to monitor autophagic activity [42]. In the present study, lgg-1::GFP puncta were evaluated to compare autophagy in C. elegans according to the anesthetic exposure and erythropoietin pretreatment. It was rarely observed at adult stages in isoflurane-unexposed animals. However, in the repeated isoflurane-exposed and erythropoietin-pretreated worms, an increase in the number of lgg-1::GFP puncta and large aggregates of lgg-1::GFP in the seam cells was observed that may reflect the accumulation of autophagosomes. Both isoflurane and erythropoietin seemed to induce autophagy in C. elegans; however, we had to discriminate between either an increased autophagosome formation or reduced degradation of the autophagosome. Thus, expression of several genes related to the autophagy were evaluated.
The expression of let-363 changed significantly on repeated isoflurane and erythropoietin pretreatment. The let-363 in C. elegans that is an ortholog of the target of rapamycin kinase in humans negatively regulates autophagy induction [43]. When considering the decrease of let-363 by isoflurane and erythropoietin, repeated anesthetic exposure and erythropoietin pretreatment may activate autophagy induction. Additionally, several cellular stresses such as nutrient deficiency, hypoxia, oxidative stress, and accumulation of protein aggregates are known to induce autophagy [28]. Repeated isoflurane exposure also increased oxidative stress in C. elegans that might have contributed to autophagy induction.
However, the bec-1, atg-5, and lgg-3 levels differed significantly between the Iso and EPO-Iso groups. Although the atg-7 expression did not differ significantly between these two groups, it was increased by EPO treatment. Both bec-1 and atg-7 are related to the autophagic pathway that is involved in vesicle nucleation and expansion, respectively [44]. In the established C. elegans models having pathologic protein aggregates, such as polyglutamine aggregates or human β-amyloid peptides, bec-1 and atg-7 seem to play key roles in their lysosomal degradation and suppresses the accumulation of abnormal intracellular proteins [45,46]. Another autophagy-related gene, atg-5 protects the pathological feature of Parkinson’s disease and restores the autophagy degradation activity in the zebrafish model [47]. In the autophagy process, atg-5 acts on the autophagosome formation by forming a complex with lgg-3 that is an ortholog of ATG12 of the mammalian gene. The forced expression of these four genes was observed significantly only when C. elegans was pretreated with erythropoietin, suggesting that the autophagosome degradation was enhanced concurrently following the autophagy induction in erythropoietin-treated worms. Although autophagy was activated by repeated isoflurane exposure in the Iso group, the autophagic degradation process seemed to be inadequate compared to that in the erythropoietin-treated animals.
This study had several limitations. First, autophagy was not observed sequentially in the course of the multiple anesthetic exposure. Instead, we compared the final status of autophagosome accumulation and autophagy-related gene expression in each group. Thus, the autophagy-related markers according to the AIN and erythropoietin treatment were limited. It was further difficult to determine whether this was due to cumulative damage, long-lasting effects, or delayed reactions to repetitive isoflurane exposure. Second, only one inhalation anesthetic gas was applied to the worms and the isoflurane concentration was higher than the clinical concentration. The protective effect of erythropoietin on the AIN was found in this experimental setting; however, it is necessary to elucidate whether the same effect can be drawn in other anesthetic agents. Finally, as experimental research, all experiments were performed in a C. elegans model. Although it is good to find the genetic expression and protein levels, the clinical evidence was not certain and that requires further clinical research.
In conclusion, erythropoietin showed neuroprotective effects against AIN and modulated autophagic pathways in C. elegans. This experimental evidence of erythropoietin-related neuroprotection against AIN may be associated with the induced autophagic degradation process that was sufficient for handling enhanced autophagy induction.
Notes
Funding
This study was supported by grant no. KSA-2017-001 from the Korean Society of Anesthesiology Research Fund.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
Author Contributions
Bon-Wook Koo (Data curation; Formal analysis; Investigation; Writing – original draft)
Hyun-Jung Shin (Data curation; Investigation; Methodology; Writing – review & editing)
Sooyoung Jeon (Data curation; Formal analysis; Writing – review & editing)
Jung Hyun Bang (Data curation; Formal analysis; Investigation; Writing – review & editing)
Sang-Hwan Do (Methodology; Project administration; Supervision; Writing – review & editing)
Hyo-Seok Na (Conceptualization; Data curation; Funding acquisition; Methodology; Project administration; Writing – review & editing)
Supplementary Material
Chemotaxis index according to the erythropoietin concentration. *P < 0.001 compared to the control group; †P < 0.001 compared to the Iso group.