Korean clinical practice guidelines for diagnostic and procedural sedation
Article information
Abstract
Safe and effective sedation depends on various factors, such as the choice of sedatives, sedation techniques used, experience of the sedation provider, degree of sedation-related education and training, equipment and healthcare worker availability, the patient’s underlying diseases, and the procedure being performed. The purpose of these evidence-based multidisciplinary clinical practice guidelines is to ensure the safety and efficacy of sedation, thereby contributing to patient safety and ultimately improving public health. These clinical practice guidelines comprise 15 key questions covering various topics related to the following: the sedation providers; medications and equipment available; appropriate patient selection; anesthesiologist referrals for high-risk patients; pre-sedation fasting; comparison of representative drugs used in adult and pediatric patients; respiratory system, cardiovascular system, and sedation depth monitoring during sedation; management of respiratory complications during pediatric sedation; and discharge criteria. The recommendations in these clinical practice guidelines were systematically developed to assist providers and patients in sedation-related decision making for diagnostic and therapeutic examinations or procedures. Depending on the characteristics of primary, secondary, and tertiary care institutions as well as the clinical needs and limitations, sedation providers at each medical institution may choose to apply the recommendations as they are, modify them appropriately, or reject them completely.
Introduction
Purpose, levels, and continuity of sedation
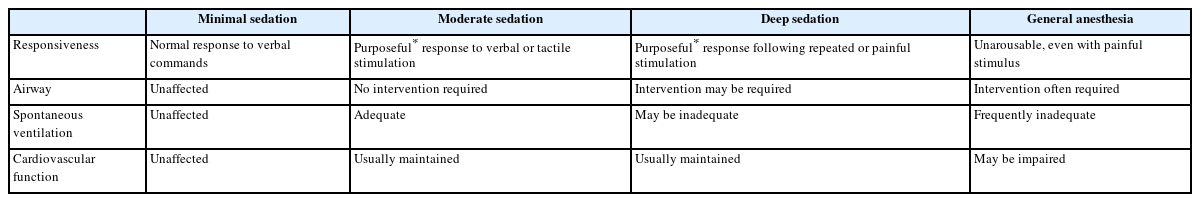
The purpose of sedation is to reduce or minimize patient discomfort, anxiety, fear, and pain associated with diagnostic tests or therapeutic procedures, thereby allowing scheduled examinations and treatments to be safely and effectively performed. Sedation is classified into four levels according to the patient’s response to verbal commands or pain-inducing stimuli, adequacy of airway maintenance and spontaneous ventilation, and maintenance of cardiovascular function as follows: mild/minimal sedation, moderate sedation, deep sedation, and general anesthesia (Table 1).
Diagnostic and therapeutic examinations and procedures are often performed under moderate sedation. Although sedation is classified into distinct and independent stages, it is a continuum, meaning patients can transition rapidly between deeper and lighter levels in the clinical setting. Additionally, accurate prediction of an individual patient’s response to a sedative is not always possible.
If a deeper level of sedation or a state of general anesthesia beyond the intended moderate sedation level is reached, the risk of cardiovascular or respiratory depression increases. Therefore, sedation providers must promptly identify and intervene to prevent serious risks and adverse events such as hypoxic brain damage, cardiac arrest, or death [1]. Conversely, insufficient sedation can lead to patient discomfort and injury, difficulties in performing scheduled procedures owing to a lack of patient cooperation, and adverse physiological or psychological reactions caused by stress. Sedation providers must respond appropriately based on the level of sedation achieved instead of the level of sedation attempted.
Safe and effective sedation depends on factors such as the choice of sedatives, sedation techniques used, experience of the sedation provider, degree of sedation-related education and training, equipment and healthcare worker availability at the healthcare institutions (primary, secondary, and tertiary care institutions) where sedation is performed, the patient’s underlying diseases, appropriate patient selection, and specific requirements and limitations of the procedure being performed.
Current status and problems associated with sedation in Korea
During examinations and procedures for diagnostic and therapeutic purposes, sedation is increasingly performed not only by anesthesiologists but also by non-anesthesiologist sedation providers from various clinical specialties. According to data from the Healthcare Big Data Hub (https://opendata.hira.or.kr/home.do), the number of prescriptions for monitored anesthesia care increased from 60,000 in 2017 to 90,000 in 2020. The absolute number of endoscopy procedures performed under sedation increased from 860,000 in 2017 to 1.57 million in 2020, and the rate of increase was even higher for sedative endoscopy procedures. As the use of sedation per year increases, the risk of adverse effects from inadequately performed sedation and, though rare, of serious complications will increase. When sedation (such as moderate sedation) is performed by non-anesthesiologists, special attention must be paid to patient safety for the following reasons. First, sedation is predominantly performed in settings outside the operating room, such as wards; outpatient departments; or emergency, computed tomography, magnetic resonance imaging, electroencephalography (EEG), ultrasound examination, endoscopy, or interventional procedure rooms, where sufficient patient monitoring devices, emergency equipment, medications, and personnel may not be as readily available as in the operating room where anesthesia professionals familiar with patient monitoring are present. Second, the quality of sedation and patient safety can vary significantly according to the systematic education and training required of the sedation providers, their experience with sedation procedures, and their degree of involvement in sedation.
An analysis of medical disputes related to anesthesia between July 2009 and June 2014 in Korea revealed that sedation accounted for 37.1% of the total cases. In sedation-related dispute cases, the majority of procedures were performed by the responsible physician administering sedation (92.3%), with insufficient attention during sedation (69.2%), failure to perform a preoperative evaluation for sedation (82.1%), absence of sedation records (89.7%), and inadequate monitoring during procedures (15.4%). The main causes of permanent damage and fatalities were respiratory events, such as hypoxemia resulting from airway obstruction or respiratory depression [2].
Preventable factors such as inadequate patient monitoring have been identified as causes of serious harm to patients undergoing procedures under sedation. Ideally, procedures conducted under sedation should be performed at a separate facility equipped with appropriate monitoring devices and emergency medications and equipment, with a dedicated healthcare team (physicians/nurses) that continuously monitors the patient’s condition.
Background for the development of sedation guidelines
One method of providing safe and effective sedation in clinical practice is the development of sedation-related practice guidelines. Several organizations have developed domestic clinical guidelines. The Korean Society of Anesthesiologists developed the Practice Guidelines for Propofol Sedation by Non-anesthesiologists in 2016 [3] and the Pediatric Sedation Guidelines (Korean) in 2017. In 2012, the Korean Society of Emergency Medicine developed the Korean Guidelines for Pediatric Procedural Sedation and Analgesia (Korean) [4]. In addition, the Korean Academy of Dental Science developed the Clinical Practice Guidelines for Dental Sedation for General Practitioners (Korean) in 2015. However, officially recognized evidence-based multidisciplinary clinical practice guidelines for sedation have not been developed in South Korea. The Korean Society of Anesthesiologists, therefore, recognized the need to develop evidence-based multidisciplinary clinical practice guidelines for sedation performed by anesthesiologists and non-anesthesiologists.
To encourage the participation of stakeholders involved in sedation, the Korean Society of Anesthesiologists proposed the development of evidence-based multidisciplinary clinical practice guidelines for sedation to representative academic societies, such as the Korean Association of Internal Medicine, Korean Society of Radiology, Korean Academy of Dental Science, Korean Society of Plastic and Reconstructive Surgeons, Korean Ophthalmological Society, and Korean Pediatric Society. On August 12, 2019, members of these societies and the Clinical Practice Guidelines Committee of the Korean Society of Anesthesiologists discussed the direction of the development of multidisciplinary clinical practice guidelines. Consequently, under the leadership of the Korean Society of Anesthesiologists, the Korean Academy of Dental Science, Korean Society of Radiology, and Korean Ophthalmological Society participated in the development process, while the remaining societies were involved in external expert reviews after the guidelines were drafted. This laid the foundation for the development of these multidisciplinary clinical practice guidelines.
Purpose and scope of these clinical practice guidelines
Purpose
The purpose of these clinical practice guidelines is to provide multidisciplinary evidence-based recommendations that assist both anesthesia providers specializing in moderate sedation and non-anesthesia providers in decision making to provide safe and effective moderate sedation to patients at medical institutions (primary, secondary, or tertiary). This is achieved by offering clear levels of evidence and a balanced risk-benefit analysis of each topic. Information on patients’ values and preferences are also included in these guidelines to assist providers with decision-making for effective sedation. However, before applying individual recommendations, sedation providers should exercise their judgment based on the specific circumstances of their medical institutions. Additionally, this study aims to provide information to providers on the benefits of performing safe sedation while minimizing anxiety and pain during diagnostic testing or procedures. Finally, these guidelines aim to enhance patients’ and healthcare policy experts’ understanding of sedation practices. Therefore, the proposed guidelines are not mandatory. Depending on the characteristics of primary, secondary, and tertiary care institutions as well as the clinical needs and limitations, sedation providers at each medical institution may choose to apply the recommendations as they are, modify them appropriately, or reject them. These guidelines are also not intended to replace the current sedation-related policies at each medical institution.
Intended users
The expected users of these clinical practice guidelines include sedation providers in primary, secondary, and tertiary care institutions who provide moderate sedation (as defined in Table 1) for diagnostic and therapeutic examinations or procedures (including outpatient and inpatient procedures); patients who receive sedation; and policy experts.
Target populations covered
These clinical practice guidelines are intended to target adult and pediatric patients undergoing moderate sedation for diagnostic or therapeutic examinations or procedures (including outpatient and inpatient procedures). According to the age guidelines of the Korean Pediatric Society, pediatric patients are individuals aged < 19 years, excluding newborns.
Scope
These guidelines focus on moderate sedation before, during, and after diagnostic and therapeutic examinations or procedures. As the risk of transitioning from moderate to deep sedation varies depending on the medications used and individual patient characteristics, guidelines on monitoring for and managing complications that may arise from deep sedation, such as cardiovascular and respiratory depression, are included. These clinical practice guidelines comprise 15 key questions covering various topics related to the following: the sedation providers (including their education); medications and equipment available, appropriate patient selection; anesthesiologist referrals for high-risk patients; pre-sedation fasting; comparison of representative drugs used in adult and pediatric patients; respiratory system, cardiovascular system, and sedation depth monitoring during sedation; management of respiratory complications during pediatric sedation; and discharge criteria. These guidelines do not cover topics such as premedication before general anesthesia, local anesthesia during sedation, sedation without accompanying diagnostic or therapeutic procedures, or minimal sedation. These guidelines also do not address general anesthesia.
Determining the level of evidence and grade of recommendation
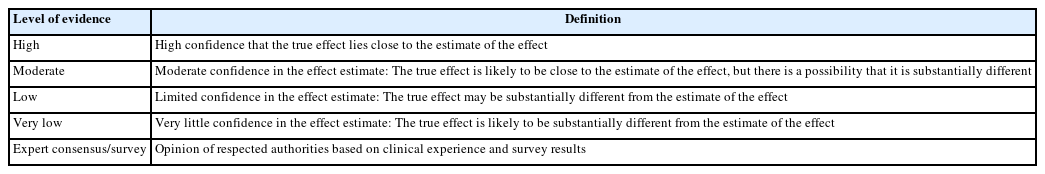
A modified Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was adopted to assign the level of evidence and grade of recommendation [5]. The levels of evidence are listed in Tables 2 and 3 presents the grades of recommendation based on several rounds of discussion. When the current research available was insufficient to evaluate the level of evidence, experts’ opinion surveys of sedation providers were collected (261 respondents). Final version of recommendation was confirmed by two-rounds voting of the committee, user opinion survey (120 respondents) and 5 external experts’ review.
Guidelines
Key Question 1. Does periodic formal training of sedation providers improve patient safety?
Recommendation: Sedation providers are recommended to undergo periodic formal training regarding sedation to ensure patient safety.
Recommendation level: Elective use (Do, conditional)
Level of evidence: Expert consensus/survey
Background: A systematic approach to education, supervision, and credentialing can facilitate safe practice of sedation [6]. However, individual healthcare institutions and departments have implemented their own education and training procedures, with variability in the quantity and quality of education and training among individual sedation providers. Therefore, the need for sedation providers to have access to periodic, comprehensive, and systematic education and training for safe and efficient sedation administration is increasing [7].
Evidence summary: Relevant research on whether regular education for sedation providers improves patient safety is lacking. This may be due to the ethical issue of administering sedation without conducting education rather than due to the need for the research itself. As the current research available was insufficient to evaluate the level of evidence, expert opinion surveys of sedation providers were collected. Recommendations were based on the survey results, and the level of evidence was evaluated using expert consensus surveys.
To ensure that patients undergoing procedures experience no distress and that an appropriate level of sedation is achieved without limitations, sedation providers must have sufficient knowledge and training. A significant majority of the respondents to the expert opinion survey agreed (strongly agreed: 76.6%, agreed: 20.7%) that sedation management conducted by appropriately trained sedation providers who have received adequate education can reduce the occurrence of complications and improve effective sedation and pain management, thus enhancing patient safety. The opinion that regular sedation education for sedation providers is feasible in the domestic medical setting was supported by the majority of respondents (strongly agreed: 15.7%, agreed: 41.8%). Regarding the frequency of sedation education for sedation providers, the most common opinion was a two-year cycle (41%), followed by a three-year cycle (28.7%), a cycle of five years or more (12.3%), and a cycle of less than one year (11.9%). Periodic sedation-related education on the characteristics of patients receiving sedation and essential techniques, medications, and monitoring systems is expected to improve providers’ capacity to achieve and maintain appropriate and safe sedation in patient care and treatment settings.
During the decision-making process for recommendations, most members (88.2%) of the attending committee expressed support for the recommended direction in terms of periodic sedation-related regular education recommendations, and all attending members (100%) supported the recommended levels. According to the review by external experts, 80% of them expressed support for the recommendations (strongly agree 40%; agree 40%), and in the user survey, 89.2% expressed support for the recommendations.
Key Question 2. Does performing sedation procedures in a standardized environment appropriately equipped with medications and devices necessary for sedation help to effectively manage sedation and pain and reduce complications?
Recommendation: When administering sedation, a standardized environment equipped with the minimum medications and devices necessary for sedation is recommended for proper management of the patient and to reduce the incidence of complications due to sedation.
Recommendation level: General use (Do, strong)
Level of evidence: Expert consensus/survey
Background: In healthcare settings, the demand for procedures performed outside the operating room is increasing, leading to a growing interest in and demand for sedation and pain management. Sedation and analgesia are sometimes administered by the physicians performing the procedure rather than anesthesiologists. To ensure patient safety, sedation should be performed in an easily accessible and standardized environment with the necessary medications and devices available [8]. The American Society of Anesthesiologists (ASA) also emphasizes the highest level of quality regarding the structural aspects of drug storage and administration and occupational safety during sedation performed in healthcare settings outside the operating room [9].
Evidence summary: No research is currently available on whether providing sedation in an environment equipped with the necessary medications and equipment improves patient safety. Owing to insufficient research to conduct an evidence-based assessment, an expert opinion survey was distributed to experts in sedation-related fields and the results were compiled. Recommendations were made based on the survey results, and the final level of evidence was evaluated through an expert consensus survey.
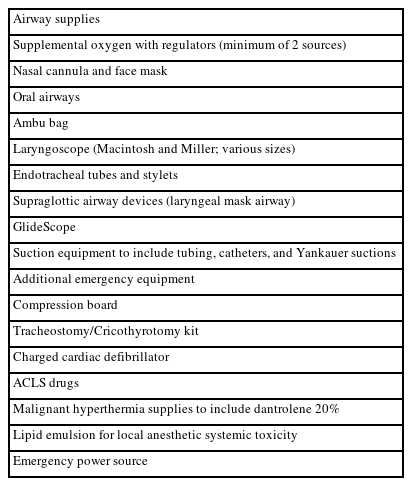
With the increase in demand for sedation performed outside the operating room (e.g., outpatient or clinical settings), the need for adequate monitoring of vital signs and management of side effects, complications, and emergency situations outside the operating room has increased. The importance of preventing side effects and complications during sedation is also emphasized. According to the expert survey results, the majority of respondents agreed (strongly agreed: 75.5%, agreed: 22.2%) that performing sedation in an environment equipped with the necessary medications and equipment is effective for managing sedation and pain management and reducing complications. Therefore, providing a consistent working environment for sedation providers that is at least minimally equipped with essential emergency and airway-related equipment and medications is expected to allow for appropriate monitoring of vital signs as well as improve providers’ capacity to handle emergency situations, minimize side effects, and prevent or reduce complications. The minimal essential equipment and drugs necessary for sedation are listed in Table 4.
Therefore, providers are recommended to perform sedation in an environment with appropriate medications and equipment to ensure patient safety. By consistently administering sedation in a systematic work environment equipped with the necessary medication and equipment, proficiency with managing sedation-related situations can be improved, leading to more effective management and prevention of sedation-related side effects and complications.
If a separate space can feasibly be allocated for sedation it should be equipped with the minimal medications and equipment necessary for sedation, and procedures are recommended to be performed under sedation in that space. However, if providing a separate space is not feasible owing to the conditions of the medical institution, providers are recommended to equip the area where the procedure is to be performed with the medications and equipment necessary for sedation to establish a minimal and systematic working environment.
More than 70% of the attending committee members supported the recommendation levels and directions, and there were no dissenting opinions regarding the content of the recommendations. According to the results of the public hearing, the approval rate for the recommended directions was 81.3%, and the approval rate for the recommendation levels was 76.5%. The external experts’ review also showed an approval rate of 80%.
Key Question 3. Do criteria need to be established for selecting eligible patients for moderate sedation?
Recommendation: Sedation providers are recommended to establish criteria for selecting eligible patients for moderate sedation based on the environment of each healthcare institution and to implement sedation accordingly.
Recommendation level: General use (Do, strong)
Level of evidence: Expert consensus/survey
Background: Although moderate sedation can be administered to any patient, it is safest for healthy patients. Healthy patients are classified as having an ASA status of Ⅰ or Ⅱ in the physical status classification system. Patients are classified as having an ASA status of Ⅰ or Ⅱ if they meet the following criteria: no serious behavioral problems, no severe gastroesophageal reflux, or no upper respiratory tract infections; no anticipated difficult airway; and no allergy to the administered drug. Previous studies have assessed moderate sedation in patients with an ASA status of Ⅰ or Ⅱ [10–15]. However, sedation must be performed carefully in elderly, and obese, and pregnant patients [12,15]. Adverse effects, including unintended deep sedation, hypotension, and hypoxia, have been reported in patients who are older or obese; those with an ASA status of Ⅲ or higher, with airway problems (e.g., sleep apnea and respiratory distress syndrome), with cardiovascular risks, with allergies, or with difficulty cooperating such as pediatric patients; those receiving psychiatric medications (including benzodiazepines); and those with a history of gastric bypass surgery (Table 5) [1,16–23].
Evidence summary: Few studies have directly compared the benefits and potential risks associated with implementing patient selection criteria for moderate sedation in terms of patient safety. As the level of evidence could not be evaluated due to insufficient research, an expert opinion survey of sedation-related experts was collected, and recommendations were determined based on the survey results and expert consensus.
The majority (91.2%) of respondents reported that establishing patient selection criteria for sedation is helpful for preventing complications (strongly agree: 52.5%, agree: 38.7%). Furthermore, the majority of respondents (95.4%) agreed that defining the criteria for consulting an anesthesiologist to manage sedation for patients undergoing moderate sedation would help to prevent complications (strongly agree: 63.6%, agree: 31.8%). Approximately one-fourth (24.5%) of the respondents indicated that an ASA status of Ⅰ should require referral to an anesthesiologist, 29.1% indicated that a referral should be made for patients with an ASA status of Ⅱ, and 46.4% reported that patients with an ASA status of Ⅲ or higher should be referred to an anesthesiologist. Therefore, 24.5% of the surveyed experts believed that all sedated patients should be referred to an anesthesiologist, 53.6% believed that patients classified as ASA Ⅱ or higher (ASA Ⅰ + Ⅱ) should be referred to an anesthesiologist, and 96.1% believed that patients classified as ASA Ⅲ or higher (ASA Ⅰ + Ⅱ + Ⅲ) should be referred to an anesthesiologist.
The first round of voting showed an agreement rate of over 70% for both the direction and level of the recommendation. In an internal user opinion survey, it received a 91.5% approval rate. However, in an external expert opinion survey, the recommendation received a 60% approval rate.
Key Question 4. For adult patients, is the incidence of sedation-related complications reduced when sedation is performed by an anesthesiologist rather than a non-anesthesiologist?
Recommendation: For adult patients, providers are recommended to perform sedation under the supervision of an anesthesiologist that specializes in pain management to prevent unintended deep sedation and ensure patient safety.
Recommendation level: Elective use (Do, conditional)
Level of evidence: Low
Conditions: Selective use of sedation performed by anesthesiologists is recommended for patients with an ASA physical status of Ⅲ or higher, those with an anticipated difficult airway or with previous difficulties with anesthesia or sedation, and those undergoing complex or invasive procedures.
Background: The types of specialists that provide sedation vary according to the healthcare environment and medical regulations in each country, and opinions on the scope of sedation provided by non-anesthesiologists differ across clinical guidelines. According to most clinical practice guidelines for gastrointestinal endoscopic sedation, patients with a high ASA status or difficult airway management have a higher incidence of cardiopulmonary complications and rate of mortality with complex and highly invasive procedures. Therefore, to ensure safety, anesthesiologists are recommended to perform the sedation. This guideline was initially intended to confirm whether there were differences in the risks related to sedation according to the provider (anesthesiologist vs non-anesthesiologist). However, most randomized controlled trials comparing the safety of sedation performed by an anesthesiologist versus a non-anesthesiologist included low-risk patient groups (mostly patients with ASA status of Ⅰ and Ⅱ and some patients with an ASA status of Ⅲ), and only a few studies included high-risk patients. Therefore, most clinical practice guidelines for low-risk patients that have been developed were based on the results of a meta-analysis of randomized controlled trials targeting low-risk patients with an ASA status of Ⅰ or Ⅱ.
Evidence summary: Through a literature search and screening process, three randomized controlled trials were identified, including 635 patients, to analyze this key question [24–26]. Among them, 330 patients were assigned to the non-anesthesiologist sedation provider group and the remaining 305 were assigned to the anesthesiologist group. In all three studies, the incidence of sedation-related hypoxemia (non-invasive oxygen saturation [SpO2] < 90% or 85%), need for interventions to improve ventilation (head tilt or chin lift, mask ventilation assistance, increased inhaled oxygen concentration), and frequency of hypotension and bradycardia were measured.
The incidence of hypoxemia (risk ratio [RR]: 0.85, 95% CI [0.50, 1.44]) and the need for airway management interventions (RR: 0.75, 95% CI [0.47, 1.21]) during sedation were not significantly different when sedation was performed by an anesthesiologist than by a non-anesthesiologist.
In a randomized controlled trial conducted by Park et al. [26], no significant difference was noted in the overall incidence of sedation-related complications among the 154 patients with an ASA status of Ⅰ or Ⅱ who underwent sedation by an anesthesiologist or non-anesthesiologist. However, the occurrence of unintentional deep sedation (MOAA/S 0–2) was higher in the group that underwent sedation by a non-anesthesiologist than in the group that underwent sedation by an anesthesiologist (5.1% vs. 17.1%, P = 0.018). Satisfaction with the sedation provider was high among patients who underwent sedation by an anesthesiologist (P = 0.001). Patient-reported satisfaction was not significantly different between the groups (odds ratio [OR]: 1.48, 95% CI [0.45, 4.91]). Additionally, no significant differences were noted in the incidence of hypotension or bradycardia between the groups in the meta-analysis. In two of the included studies [24,26], physicians in the non-anesthesiologist group performed sedation independently of the operating physician and had experience providing sedation. Therefore, undergoing sedation by an anesthesiologist did not reduce the occurrence of sedation-related complications in patients included in the analysis with a low risk of complications (ASA status of Ⅰ or Ⅱ). However, most patients with an ASA status of Ⅰ or Ⅱ underwent procedures for less than 40 min, and the frequency of unintentional deep sedation was significantly reduced. Therefore, these results may not be applicable to patients who undergo complicated and invasive procedures or high-risk patients.
Ferreira et al. [24] reported that the recovery time was approximately 10 min longer when sedation was performed by an anesthesiologist (58 ± 33 vs. 67 ± 29 min, P = 0.032); however, Park et al. [26] reported no significant difference between the two groups in terms of the recovery time. Guerra et al. [25] reported that patients who received sedation from an anesthesiologist using propofol had a longer total hospital stay than patients who received sedation from a non-anesthesiologist using midazolam (31 vs. 28 h, P = 0.003), resulting in higher total hospitalization costs (677 vs. 562 euros, P = 0.001). Although the cost is expected to vary depending on the type of healthcare expenses and procedures conducted in each country, the use of an anesthesiologist can increase the cost of the hospital stay.
According to the results of our meta-analysis, in adult patients, sedation performed by an anesthesiologist was not found to reduce the frequency of significant complications such as hypoxemia. Additionally, no significant differences were noted in the total number of sedatives used or recovery time, although undergoing sedation by anesthesiologists may result in additional costs associated with hospitalization. The balance between the benefits and harms can be judged according to the characteristics of the patient and the procedure. In low-risk patients undergoing short and simple procedures, the financial burden may be higher; however, even in low-risk patients, the possibility of unintentional deep sedation cannot be completely ruled out. If respiratory depression lasts longer than a few minutes, serious complications, such as hypoxic organ damage or subsequent cardiac arrest or death, may occur.
A study analyzing 105 medical dispute cases in South Korea between 2009 and 2014 reported that 92.4% of disputes were related to sedation performed by non-anesthesiologists. Even if the incidence of sedation-related complications is low in patients in the low-risk group, a serious risk of complications (e.g., hypoxic brain damage, organ damage, cardiac arrest, or death) resulting from respiratory depression secondary to unintentional deep sedation is still present.
Patients and caregivers prefer to receive sedation from an anesthesiologist with specialized knowledge and skills in airway management, hemodynamic monitoring, and emergency responses. When asked, “Do you think a skilled sedation provider who only manages the sedation is necessary for patient safety and successful completion of procedures?”, most patients who had undergone sedation (94%; 43/46 patients) reported that a skilled sedation provider should monitor sedation. When asked, “If the patient’s condition is critical, do you think that sedation by an anesthesiologist will improve patient safety?”, 87% (40/46) of the respondents responded “yes,” and the remaining 13% (6/46) reported that they did not know. According to the results of this survey, patients undergoing sedation prefer to be monitored by a skilled anesthesiologist, especially when the patient’s condition is critical.
Sedation can be safely performed in patients with an ASA status of Ⅰ or Ⅱ by a non-anesthesiologist with appropriate monitoring. However, the type of procedure and degree of invasiveness must be considered. Patients undergoing complicated, long, or painful procedures that require deep sedation; those with a history of anesthesia or sedation complications; those with an ASA status of Ⅲ or higher; and elderly patients is recommended to undergo sedation performed by an anesthesiologist to ensure that deep sedation is prevented, the airway is properly managed, and appropriate emergency responses are performed (Table 6).
Consensus on the recommendation level and direction was reached among all participating members of the development committee for patients with an ASA status of Ⅲ or higher or for high-risk patients with suspected airway management complications. A total of 91.4% of the respondents to the user opinion survey of the draft clinical practice guidelines agreed with the recommendations. However, external expert reviews had a low agreement rate (20%). Therefore, future research must be conducted to provide a clearer definition of high-risk groups and to compare the incidence of complications between high-risk patient groups that undergo sedation performed by anesthesiologists and non-anesthesiologists. However, because of the lack of prospective comparative literature that directly compares sedation-related complications when sedation is performed by an anesthesiologist versus a non-anesthesiologist in high-risk patients, the level of evidence for this practice guideline is low. As most previous research has been conducted on low-risk patients, accurately comparing the incidence of sedation-related complications between different high-risk groups is challenging. However, sedation performed by an anesthesiologist skilled in rapid airway maintenance and hemodynamic management would improve the safety of sedation.
Key Question 5. Is fasting before the procedure necessary for adult patients undergoing sedation?
Recommendation: Adult patients scheduled for sedation are advised to fast from clear fluids for two hours and from solid foods for six hours prior to the procedure.
Recommendation level: Elective use (Do, conditional)
Level of evidence: Very low
Conditions: Fasting criteria for patients undergoing sedation should be comprehensively analyzed based on the urgency of the procedure and the depth of sedation.
Background: Sedation is used to facilitate various procedures. Although pulmonary aspiration is rare, it can result in considerable harm or even death. The aim of this clinical practice guideline is to prevent pulmonary aspiration. Although pulmonary aspiration related to general anesthesia has been extensively studied, only a few studies and publications have reported pulmonary aspiration during sedation. Most previous studies in the field of gastroscopy have been case reports or retrospective audits. Strategies to prevent pulmonary aspiration during sedation are similar to those traditionally recommended for general anesthesia.
Evidence summary: Studies related to fasting (including fasting time) in adult patients undergoing sedation were identified through a literature search. Review articles, treatment guidelines, and retrospective studies were excluded. As few studies directly comparing the benefits and harms of fasting were identified, one randomized controlled trial and four non-randomized observational studies on patient risk factors for complications were included in the meta-analysis.
Bell et al. (2007) [27] compared the safety of fasting and non-fasting conditions. However, only the percentage of aspiration, adverse respiratory events, and respiratory interventions, were described; no blinded assessment of outcomes was provided. Koeppe et al. (2013) [28] compared the ease of gastroscopy between fasting and non-fasting patients, although all conditions in the study were considered fasting according to the ASA guidelines. Overnight fasting was compared with fasting from liquids and solids for two hours prior to the procedure in terms of aspiration prevention (benefit) and hunger, weakness, anxiety, and thirst (harms). This study presented only indirect evidence and was therefore considered an observational study. Fasting from liquids for two hours prior to sedation was found to cause patient harm [28]. Hamid [29] and Davies et al. [30] compared the results of surveys conducted after the second and third education sessions. However, the sample sizes were small, and the number of samples was not accurate, as only percentages or average scores were presented. In addition, after the second and third education sessions, all patients fasted according to the ASA guidelines, and only indirect evidence was presented. Finally, as the researchers were not blinded, no blinded assessment of the outcomes was performed in any of these studies. All the studies analyzed cases of participants fasting according to the ASA guidelines, even after the second and third surveys, and analyzed the harm resulting from fasting before sedation. In another study by Manchikanti et al. [31], all patients underwent procedures without fasting, and the incidences of aspiration and vomiting were simply described. Overall, the bias of the study design was low; however, owing to the very small sample size, low incidence of complications, and the inconsistency and indirectness, this recommendation was determined to have a very low level of evidence.
Three of the five studies included in a previous meta-analysis reported no observation of pulmonary aspiration [27,28,31]. Only a few studies have reported pulmonary aspiration during sedation. Based on case reports from 1985 to 2016, only nine cases of aspiration during sedation have been reported, excluding those that occurred during gastrointestinal endoscopy [32–36]. However, estimating the incidence of aspiration during sedation is difficult. Among these reported cases, one death caused by pulmonary aspiration during non-gastroscopic sedation was reported [32]. Fasting from solids for at least 4–6 h and from liquids for at least 2 h were reported in all nine case reports. The patients had significant underlying diseases and propofol was the main sedative administered. The results suggest that fasting does not completely prevent pulmonary aspiration.
One prospective non-randomized observational study described the frequency of sedation-related adverse effects based on fasting status. No cases of aspiration were reported among the 400 procedures performed in the emergency department. Respiratory complications occurred in 19.5% of patients who fasted and 22.4% of patients who did not fast. Respiratory interventions were required in 24.6% of the patients who fasted and 33.3% of the patients who did not fast. Vomiting occurred in 0.8% of patients who fasted and in 0.4% of patients who did not fast [27]. Among the 18,472 interventional pain treatment procedures performed under sedation in 3,179 patients, aspiration did not occur, but respiratory depression occurred in two cases [31]. The patients in this study consumed solids for up to 2 h prior to the procedure and liquids for up to 15 min prior to the procedure. Antiemetics were required in 15.4% of the procedures based on previous episodes of nausea and vomiting. Nausea was reported in 1.6% of the procedures, and vomiting occurred in three cases. There was no conclusive or direct evidence supporting the requirement for fasting.
One randomized controlled trial and four observational studies were included in the meta-analysis of the harms of fasting. Fasting from clear liquids for up to 2 h prior to gastroscopy resulted in anxiety in 12% of patients, hunger in 44%, and weakness in 22% [28]. When liquid intake was permitted until 2 h prior to sedation, subjective dehydration was reported in 25% of patients, and clinical and objective dehydration was observed in 25% of patients [30].
Based on the available evidence, we cannot conclude that fasting from clear liquids for 2 h and from solid food for 6 h prior to a procedure requiring sedation reduces sedation-related complications. However, aspiration may result in serious complications, and fasting is believed to reduce the gastric contents, thus minimizing the potential harm that could result from aspiration. In contrast, fasting can cause temporary discomfort, including hunger, anxiety, lethargy, thirst, and dehydration. The harm caused by fasting may be partially alleviated if liquids are permitted until 2 h prior to the procedure.
All members in attendance agreed with the level and direction of these recommendations, and none disagreed with the contents of this clinical practice guideline. In a user opinion survey of the draft of this clinical practice guideline, 94.9% of the anesthesiologists agreed with the recommendation. In the external review, 20% of the experts strongly agreed, 60% agreed, and 20% strongly disagreed with this clinical practice guideline.
Key Question 6. Are there any differences in the efficacy and safety of intravenous propofol versus midazolam monotherapy in adult patients undergoing moderate sedation?
Recommendation: The use of intravenous propofol for sedation in adult patients results in a significantly shorter recovery time than that of intravenous midazolam. Both drugs are currently widely used in clinical practice. The choice of sedative can be selected based on the duration of the examination or procedure, experience of the sedation provider, and healthcare environment.
Recommendation level: Elective use (Do, conditional)
Level of evidence: Moderate
Conditions: Propofol can be used to shorten the recovery time, and midazolam can also be used to shorten the recovery time when combined with flumazenil.
Background: Commonly used sedative drugs include propofol, midazolam, ketamine, and dexmedetomidine. These medications are also used in combination with other sedatives and narcotic agents based on the clinical situation, examination or procedure time, and level of stimulation. For this guideline, the efficacy and safety of propofol versus midazolam monotherapy, both of which are widely used to achieve moderate sedation in adult patients, were compared. Studies that combined these sedatives with narcotic preparations were excluded from the analysis.
Evidence summary: The benefits of propofol compared to midazolam were examined in six prospective randomized studies. These analyses primarily focused on efficacy (patient satisfaction) and safety (frequency of hypoxemia and hypotension and recovery time).
No significant difference in the incidence of hypoxemia (oxygen saturation < 90%) during sedation was noted between the propofol and midazolam groups in the meta-analysis (RR: 0.88, 95% CI [0.56, 1.37]) [37–39]. The lowest peripheral oxygen saturation reported during the procedure was significantly lower when propofol was administered than when midazolam was administered (mean difference [MD]: −1.55%, 95% CI [−2.75, −0.35]) [37,40]. As the average minimum peripheral oxygen saturation exceeded 90% for both drugs, it was difficult to determine which drug was superior in preventing hypoxemia.
One of the six selected studies evaluated the frequency of hypotension during sedation. The meta-analysis results showed no significant difference in the incidence of hypotension (systolic blood pressure ≤ 90 mmHg) between the propofol and midazolam monotherapy groups (RR: 0.75, 95% CI [0.14, 4.13]) [39].
Four of the included studies evaluated the time required to recover consciousness after the completion of the procedure. The recovery time was significantly shorter after propofol than after midazolam administration (MD: −8.88 min, 95% CI [−10.45, −7.32]) [38,39,41,42]. Three of the included studies targeted patients with liver cirrhosis [38,39,42], and one included general patients [41]. The duration of the effect of propofol was shorter than that of midazolam due to the large distribution volume and short distribution half-life of propofol [43,44]. Both propofol and midazolam are metabolized by the liver. Although the pharmacokinetics of propofol are not significantly affected by cirrhosis [45], the pharmacokinetics of midazolam are dependent on liver function, resulting in a prolonged elimination half-life and a potentially longer duration of action in patients with liver cirrhosis [46]. Thus, compared to propofol, the recovery time for midazolam may be relatively delayed in patients with liver cirrhosis [38,39,42]. However, even in general patients, the recovery time with midazolam was significantly longer than that with propofol [41]. Patient satisfaction was similar for propofol and midazolam (RR: 0.38, 95% CI [−0.59, 1.36]) [38,42].
In our meta-analysis of studies on adult patients undergoing moderate sedation, the average recovery time was 8.88 min shorter when propofol was used than when midazolam was used. However, the incidence of hypoxemia, hypotension, and patient satisfaction were not significantly different between the groups. Propofol and midazolam are widely used for sedation in primary, secondary, and tertiary care institutions, and the views and preferences of sedation providers vary. However, the recovery time is longer with midazolam than with propofol. Therefore, patients should be provided with sufficient recovery time and caution should be exercised when administering midazolam.
In addition to midazolam and propofol, various sedatives and narcotics, including ketamine and dexmedetomidine, are currently used for sedation. Sedatives should be selected and used in appropriate combinations based on factors such as the time required for the examination or procedure, level of stimulation, experience of the provider, and the healthcare environment. A drug can be used alone or in combination with other drugs. When sedatives and narcotic analgesics are administered concurrently, the synergistic effects of sedation and analgesia require careful dose adjustments.
In the external expert survey regarding this clinical practice guideline, no significant differences or additional opinions were noted (strongly agree: 20%, agree: 80%). In the user opinion survey, 98.3% of respondents agreed with the final version of this clinical practice guideline.
Key Question 7. Is oral chloral hydrate safer than oral midazolam for sedation of pediatric patients?
Recommendation: As no significant differences have been reported on the safety and efficacy of oral chloral hydrate versus midazolam for sedation in pediatric patients, oral chloral hydrate can be used based on the patient’s condition and availability of the medication.
Recommendation level: Elective use (Do, conditional)
Level of evidence: Very low
Background: Oral sedation is widely used as an easy, safe, and inexpensive method of drug-assisted sedation in pediatric patients. Chloral hydrate, which is mainly used for oral sedation and is classified as a sedative-hypnotic, induces sleep in pediatric patients. When chloral hydrate is administered orally, its medicinal effect begins within 15–30 min and the maximum effect occurs after an hour and lasts > 5 h. In the United States, chloral hydrate production was discontinued in 2012, which limited its use. In addition, no antagonist is available for chloral hydrate. In pediatric patients, midazolam is rapidly cleared and oral midazolam is promptly absorbed from the gastrointestinal tract, reaching peak plasma concentrations within one hour. Midazolam can produce a paradoxical reaction, including agitation, irritability, hyperactivity, rage, and hostility toward caregivers. Impaired memory, primarily anterograde amnesia, in which an individual is unable to recall events experienced during the period of drug action, is a potential side effect of midazolam. Flumazenil, an antagonist, can be administered to reverse the effects of midazolam. The key question for this recommendation was the safety and efficacy of oral chloral hydrate versus oral midazolam for sedation in pediatric patients.
Evidence summary: Among the seven selected prospective randomized controlled trials, six included benefits that could be analyzed. Our meta-analysis results showed a success rate of sedation at 93.46% (357/382) among patients administered chloral hydrate and 74.94% (302/403) among patients administered midazolam (RR: 1.26, 95% CI [0.72, 2.19], P = 0.41). Although the difference was not significant, the success rate of sedation was higher in the chloral hydrate group than that in the midazolam group. Oral chloral hydrate is used to achieve sedation in pediatric patients who have difficulty cooperating during diagnostic examinations and invasive procedures, allowing for the success of these examinations and procedures. Systematic reviews and meta-analyses evaluating the safety of chloral hydrate and midazolam for pediatric sedation have been conducted [47–53]. No major neurological or respiratory adverse effects were reported when chloral hydrate or midazolam was used for pediatric sedation. The frequency of minor adverse effects was 21.36% (91/426) among patients administered midazolam and 12.35% (50/405) among those administered chloral hydrate. Although minor adverse effects were less frequent with chloral hydrate, the difference was not significant (RR: 0.90, 95% CI [0.34, 2.41], P = 0.84).
The most frequent adverse reactions were prolonged sedation, paradoxical agitation, and gastrointestinal side effects. Oxygen saturation decreased by > 10% in one patient who received chloral hydrate, and respiratory support was required in three patients who received chloral hydrate and in one patient who received midazolam.
Chloral hydrate may be used as an oral sedative in pediatric patients. Although the success rate of sedation is higher and fewer adverse drug reactions are reported with chloral hydrate than with oral midazolam, the incidence of respiratory side effects is higher with oral chloral hydrate. Therefore, chloral hydrate should be used with caution in patients with respiratory problems.
At the first internal committee meeting, 52.9% of the members agreed with the direction of the recommendation. The contents of this clinical practice guideline were revised, resulting in a 100% agreement at the second internal committee meeting. A total of 80% of the external experts agreed with the level of recommendation of this clinical practice guideline for sedation, and 90% of the respondents to the user opinion survey agreed with the recommendations.
Key Question 8. Is ketamine safer than midazolam for sedation in pediatric patients?
Recommendation: (1) Although no significant differences in the safety or efficacy of ketamine and midazolam for sedation in pediatric patients were found, the use of oral ketamine should be limited given its lower success rate of sedation.
Recommendation level: Limited use (Do not, conditional)
Level of evidence: Very low
Conditions: When additional dosing or titration is challenging.
(2) Intravenous administration of ketamine in pediatric patients has a relatively faster time of onset but an equivalent success rate of sedation and a similar safety profile to that of midazolam; therefore, ketamine can be administered to some pediatric patients depending on the type of sedation needed, the patient’s condition, and the preference of the sedation provider.
Recommendation level: Elective use (Do, conditional)
Level of evidence: Very low
Conditions: When rapid sedation is necessary, such as in cases of primary sedation failure.
Background: The use of benzodiazepines is preferred as they allow for a desirable sedation effect and can be reversed using flumazenil. At appropriate doses, benzodiazepines do not induce respiratory depression; however, when used in conjunction with opioid analgesics or multiple types of sedatives, they can lead to dangerous complications such as apnea and hypoxemia.
In contrast, ketamine is a phencyclidine derivative classified as a dissociative anesthetic. Ketamine exhibits analgesic, amnesic, and sedative properties without the loss of protective reflexes, making it an ideal sedative. Patients who receive ketamine may appear awake in a tonic state, as they are unable to communicate, but can perform involuntary movements.
In addition, the “emergence” phenomenon, wherein patients may experience nightmares or frightening hallucinations upon awakening from anesthesia, is a specific adverse reaction associated with ketamine. Administration of midazolam immediately before awakening helps prevent the emergence phenomenon. Ketamine should be administered slowly to avoid the malignant arrhythmias associated with rapid bolus administration. Intramuscular ketamine has a similar safety profile to intravenous ketamine.
The key question addressed by these recommendations is whether the safety and efficacy of ketamine for sedation in pediatric patients is superior to those of midazolam.
Evidence summary: Through a literature search and selection process, three randomized controlled trials [54–56] with a total of 167 patients were included in the analysis. Overall, 80 and 87 patients received ketamine and midazolam, respectively. In all three studies, the commonly reported outcomes were the success rate of sedation; time to loss of consciousness; adverse drug reactions; and frequency of hypoxemia, agitation, and postoperative nausea and vomiting.
The study conducted by Rubinstein et al. [54] reported a serious risk of bias with the randomization process. The remaining studies also reported a risk of bias, although not as severe. Therefore, the overall risk of bias was high. The level of evidence for the success rate of sedation, which was the key outcome, was rated very low, as inconsistency and imprecision each reduced the level of evidence by one. The level of evidence for the time to loss of consciousness was rated low, as imprecision reduced the score by one level [5]. The level of evidence for other adverse reactions was rated very low, as inconsistency and imprecision each reduced the level of evidence by one.
The number of individual studies and total patients included in the meta-analysis was also small. Detecting significant differences in the incidence of the outcomes of interest was difficult as patients were divided into oral and intravenous administration groups and few patients were included.
Considering these factors, the strength of the evidence was determined to be low and the evidence did not fully reflect all pediatric patients undergoing sedation.
In the case of oral administration, the success rate of sedation was significantly higher with oral midazolam than with oral ketamine. In the study by Rubinstein et al. [54], sedation was administered to 68 pediatric patients (aged 1–10 years) who underwent wound closure in the emergency room, and the effects of oral ketamine (5 mg/kg) and oral midazolam (0.7 mg/kg) were compared. The success rate of sedation was significantly lower with ketamine than with midazolam (RR: 0.72, 95% CI [0.57, 0.92], I2 = 0%, P = 0.008). However, no significant differences in pain scores according to the visual analog scale were noted between the groups. Considering the small patient population and the lack of statistical significance, the clinical significance remains unclear. Conversely, a previous meta-analysis [57] reported a higher success rate of sedation with oral midazolam than with oral ketamine (RR: 1.32, 95% CI [1.07, 1.62], I2 = 0%, P < 0.01).
Oral formulations of midazolam and ketamine are currently unavailable in South Korea. Although intravenous drugs have been mixed with syrups or glucose solutions in some cases, no oral formulations have been officially approved for use. Therefore, ketamine is typically administered parenterally (via a non-oral route) in South Korea.
No significant differences were noted in the success rates of sedation between intravenous ketamine and midazolam. In the study by Thevaraja et al. [55], sedation was administered to 34 pediatric outpatients with an ASA status of Ⅰ who were undergoing a urodynamic study. Patients in the ketamine group received 0.25 mg/kg of intravenous ketamine followed by a continuous infusion at 10–20 μg/kg/min. Patients in the midazolam group received 0.02 mg/kg of intravenous midazolam followed by a continuous infusion at 1–2 μg/kg/min. Although the time to loss of consciousness was shorter in the ketamine group than in the midazolam group, the success rate of sedation was 100% in both groups. Both intravenous midazolam and low-dose intravenous ketamine provided satisfactory sedation, without affecting the urodynamic test values in pediatric patients (RR: 1.00, 95% CI [0.94, 1.07]). No studies comparing oral and intravenous ketamine were included in the meta-analysis. A systematic meta-analysis by Cheng et al. [57] reported no significant differences in the success rate of sedation and hypnosis or in the duration of sedation between oral and intravenous midazolam (P > 0.05).
Data from all three prospective randomized controlled studies were included in the comparative analysis of drug reactions between ketamine and midazolam. Intravenous administration was used in one study, whereas oral administration was used in the other two. Among the 167 patients (Younge and Kendall [56], 2001; Thevaraja et al. [55], 2012; Rubinstein et al. [54], 2016), adverse effects were observed in 23 (13.8%). When calculated per 1,000 individuals, the incidence of adverse reactions was lower in patients who received ketamine (126 per 1,000) than in those who received midazolam (156 per 1,000). However, the difference between the two groups was not statistically significant (RR: 0.81, 95% CI [0.39, 1.69], I2 = 0%, P = 0.58). Thevaraja et al. [55], in their study involving 34 participants, reported no adverse reactions in either the intravenous ketamine or midazolam groups. Due to the rare incidence of severe complications, the total number of patients in this study was determined to be insufficient to draw accurate conclusions.
Ketamine can increase salivary secretion, leading to coughing and laryngospasms in severe cases. Therefore, ketamine should either be avoided or used with caution in cases of airway procedures and upper respiratory tract infections or when dealing with uncontrolled asthma or hypertension. These effects can be mitigated by the complementary use of atropine or glycopyrrolate [58]. Although ketamine is generally recognized as a safe sedative that does not cause significant respiratory depression, apnea can occur when it is administered rapidly or in conjunction with opioids or other sedatives. In addition, ketamine can inhibit the reuptake of catecholamines, thereby increasing sympathetic nervous system stimulation and potentially causing tachycardia. Although it is safe for pediatric patients who are hemodynamically compromised or at risk of bradycardia, it should not be used in pediatric patients with increased intracranial pressure because it can increase cerebral blood flow.
Systematic meta-analyses of adverse reactions to ketamine sedation in pediatric patients have not been reported. In a systematic meta-analysis of adverse reactions to sedation with oral midazolam in pediatric patients reported by Cheng et al. [57], the incidence of adverse drug reactions was 19.57% (189/966). Bellolio et al. [58] conducted a meta-analysis of adverse reactions reported for all pediatric sedation drugs used in an emergency room and found that mild complications such as vomiting (55.5 episodes per 1,000 patients, 95% CI [45.2, 65.8]) and agitation (17.9 episodes per 1,000 patients, 95% CI [12.2, 23.7]) were the most common, whereas dangerous complications such as hypoxia (14.8 episodes per 1,000 patients, 95% CI [10.2, 19.3]) and apnea (11.0 episodes per 1,000 patients, 95% CI [3.2, 0.11]) were less common. The need for intervention with a bag-valve mask, oral airway, or positive pressure ventilation was rare (5.0 episodes per 1,000 patients, 95% CI [2.3, 7.6]). The incidence of laryngospasm was 2.9 episodes per 1,000 patients (95% CI [1.1, 4.7]) and intubation was required in 34 of the 9,136 patients.
No aspiration was reported among the 3,326 patients. Therefore, serious adverse reactions are rare during pediatric sedation with various sedatives, and mild complications such as nausea, vomiting, or nervousness are common. The incidence of hypoxemia among all pediatric sedation cases was approximately 1.5%. In a previous meta-analysis of the adverse reactions to sedation in adult patients, the most common complication was hypoxemia [59]. Although drug selection and co-administration of narcotics differ between adult and pediatric patients, the incidence of severe complications such as hypoxemia is significantly lower in pediatric patients than in adult patients. Most patients who experienced laryngospasms had received ketamine (33/34). Although ketamine is reported to be relatively safe in adult patients, pediatric patients are at risk of severe adverse reactions such as rapidly progressing hypoxemia under sedation. Therefore, sedatives should be administered at an appropriate initial dose, with additional lower doses administered under continuous monitoring. The American Society of Anesthesiology practice guidelines [1] also recommend that the effects of sedatives should be judged by allowing sufficient time for maximum effect when administering sedatives. Even when administered via a non-intravenous route (such as the oral or nasal mucosa), sufficient time should be provided for the absorption of the previous dose and observation of the maximum effect before additional doses are administered. For patients receiving intravenous drugs, the intravenous route should be maintained until the patient is no longer at risk of cardiorespiratory dysfunction. In addition, patients should be carefully monitored for complications.
However, as this meta-analysis focused only on studies of pediatric patients who received with a single sedative agent for a procedure, few studies and patients were included. The included patients were divided into oral and intravenous administration groups owing to inconsistencies in usage and low or very low levels of evidence due to inaccuracy and imprecision. Furthermore, the incidence of events corresponding to harm was low given the low number of patients, making it difficult to detect significant differences. Due to these limitations, classifying one drug as superior was not possible.
As the direction of the recommendation differed between oral and intravenous administration, less than 70% of the attending committee members agreed with the original initial proposal, which provided recommendations solely for intravenous medications. However, oral formulations of these drugs are not commercially available in South Korea. However, as they may become available in the future, recommendations for both oral and intravenous administrations were suggested in the first round of voting, and this clinical practice guideline was revised such that oral and intravenous administrations were classified separately. During the second round of voting, no disagreements regarding the content of this clinical practice guideline were reported, with 100% approval of the direction and level of recommendation. The user opinion survey results indicated an approval rate of 80.8% for the recommended direction, and the external expert review showed an approval rating of 80%. The current clinical practice guideline and level of recommendation were finalized without any additional adjustments.
Key Question 9. Is dexmedetomidine safer than midazolam for sedation in pediatric patients?
Recommendation: In pediatric sedation, dexmedetomidine is not considered as safe as midazolam, given its potential to cause hypotension and bradycardia. However, it is considered effective for deep sedation. Therefore, depending on the type of procedure and the sedative provider’s preference, dexmedetomidine can be used as an alternative to midazolam.
Recommendation level: Elective use (Do, conditional)
Level of evidence: Moderate
Conditions: Close monitoring and management of hypotension and bradycardia in appropriate settings.
Background: Dexmedetomidine has a slightly longer time to loss of consciousness, slightly delayed onset of action, and a longer half-life than other sedatives. As a strong Alpha-2 agonist, dexmedetomidine does not cause respiratory depression, and exerts analgesic effects. Indeed, dexmedetomidine use has increased in various fields as hemodynamic stability and appropriate sedation depth is maintained. Furthermore, it reduces post-procedural agitation and decreases the occurrence of emergence delirium, leading to increased satisfaction for both the operator and examiner, and has thus been gradually replacing midazolam.
Evidence summary: The use of dexmedetomidine over midazolam has increased because provider satisfaction is higher with dexmedetomidine. After a literature search and selection process, two randomized controlled trials were included in the analysis. One study compared the sedative effects of oral midazolam versus intranasal dexmedetomidine during CT examination, and another compared the results of continuous intravenous infusions of dexmedetomidine versus midazolam during EEG [60,61].
A total of 119 patients were included in the meta-analysis, 60 of whom received dexmedetomidine and 59 received midazolam. The sedation score was evaluated in both studies, while blood pressure and heart rate were evaluated in only one study [61]. The risk of bias was low for all assessments.
The level of evidence for maintenance of appropriate sedation scores (a key outcome), the incidence of side effects related to blood pressure and heart rate, and the incidence of other adverse effects were determined. The level of evidence was moderately reliable for the maintenance of appropriate sedation scores, and low for side effects related to heart rate and blood pressure as inconsistency and imprecision each lowered the level by one.
According to the meta-analysis results based on the two selected prospective randomized controlled trials, appropriate sedation scores for performing the tests, as measured by the Ramsay Sedation Scale (RSS), were higher for dexmedetomidine than for midazolam (0.13 higher, 95% CI [−0.22, 0.47]). This finding indicates a moderate level of evidence that maintaining appropriate sedation scores with dexmedetomidine can reduce the need for additional medications, minimize the incidence of side effects related to overdosing, and effectively achieve stable sedation.
However, a systematic review comparing dexmedetomidine administration methods for pediatric sedation has not yet been conducted. Indeed, established literature on dexmedetomidine administration methods in pediatric patients is lacking, making it difficult to draw accurate conclusions. Furthermore, owing to the relatively delayed onset of action and unpredictable half-life of dexmedetomidine, its potential as a standalone sedative for maintaining sedation is limited. In addition, as the hemodynamic stability results of this meta-analysis show, dexmedetomidine may induce similar side effects to midazolam, such as hypotension and bradycardia. However, although the decrease in heart rate was concerning (6.00 lower, 95% CI [−9.85, −2.15]), the decrease in systolic blood pressure was not as concerning (16 lower, 95% CI [−0.22, −0.47]). However, a 16 mmHg decrease in systolic blood pressure may be clinically significant, and thus should be considered in decision making.
Because the meta-analysis results did not provide clear evidence that dexmedetomidine is superior to midazolam in terms of safety, fewer than 70% of the participating members supported this recommendation in the first round of voting. However, considering the increased use of dexmedetomidine in clinical practice and evidence showing that dexmedetomidine is more effective at maintaining sedation depth than intravenous midazolam, the recommendation was revised to include careful monitoring to ensure patient safety as a condition. Therefore, dexmedetomidine can be used as an alternative to midazolam, depending on the type of procedure and the preference of the sedation provider. In the second round of voting, more than 70% of the participating members approved the direction and level of the recommendation that dexmedetomidine be used in a setting where careful monitoring and treatment of hypotension and bradycardia are possible. This recommendation was approved by 74.2% of respondents in the internal user opinion survey and 100% of respondents in the external expert review. After discussions with the committee and individual members, the modified recommendations were confirmed without any further changes to the direction or level of recommendation.
Key Question 10. Is it necessary to monitor and record the depth of sedation, respiration, oxygen saturation, and blood pressure and to obtain an electrocardiogram during sedation?
Recommendation: Providers are recommended to monitor and record the depth of sedation, respiration, oxygen saturation, and blood pressure during sedation. Additional electrocardiography (ECG) monitoring is recommended for patients with cardiovascular disease.
Recommendation level: General use (Do, strong)
Level of evidence: Expert consensus/survey
Background: Monitoring the patient’s condition during sedation is important for the early detection and management of respiratory and cardiovascular depression or hypoxia. Existing clinical guidelines also recommend that the patient’s level of consciousness, ventilation, oxygenation, and hemodynamics be monitored and recorded during sedation [1,62]. With this key question, evidence-based clinical practice guidelines for depth of sedation, respiration, oxygen saturation, and blood pressure monitoring in patients undergoing moderate sedation in South Korea can be developed. Expert surveys were used as references in cases where evaluation of the evidence level was not possible due to a lack of research.
Evidence summary: The selection criteria for this meta-analysis included studies related to basic monitoring and documentation during sedation. Editorials, clinical guidelines, retrospective studies, and studies with specialized monitoring (such as end-tidal carbon dioxide pressure and bispectral index) were excluded. To the best of our knowledge, no study has directly compared the benefits or harms of basic monitoring during sedation in terms of patient outcomes and complications. In addition, no study has found whether monitoring and recording sedation depth improves patient outcomes and reduces complications. As no randomized controlled trials on oxygen saturation monitoring during sedation have been reported, this meta-analysis included four observational studies with oxygen saturation monitoring during sedation using pulse oximetry, and three observational studies of oxygen saturation in two patient groups. Owing to the paucity of studies we were unable to adequately evaluate the level of evidence; thus, sedation provider opinion surveys were collected. Recommendations were made based on the survey results, and the level of evidence was an expert consensus/survey.
In the expert opinion survey, the majority of respondents agreed (strongly agree: 67.8%, agree: 27.2%) that regular monitoring of sedation depth helps to prevent excessive or shallow sedation. Regular observation of the level of consciousness and sedation depth allows for timely recognition and response when a patient transitions to minimal or deep sedation. Therefore, appropriately managing and recognizing changes in sedation depth can reduce sedation (including minimal sedation) failure, which can cause anxiety and pain and hinder the procedure, and result in respiratory depression due to oversedation.
The majority of respondents to this survey agreed (strongly agree: 79.7%, agree: 16.1%) that continuous oxygen saturation monitoring during sedation helps to reduce the incidence of complications such as respiratory depression, hypoxia, and airway obstruction. In four observational studies that measured oxygen saturation during sedation, desaturation was observed in 49% (60/122) of patients [63–66]. Another previous study reported a decrease in oxygen saturation of 5% or more among 30% of patients aged ≥ 60 years [67]. In addition, oxygen saturation decreased to ≤ 90% in 68% and 58% of healthy pediatric patients and those with cardiovascular disease, respectively. Although no significant difference was found between the two groups, oxygen desaturation was observed in more than half of the patients [68]. Similarly, a study conducted on both adults with cardiovascular disease and healthy adults showed a similar degree of oxygen desaturation in both groups, with a lowest mean oxygen saturation of 89.5% and 90.2%, respectively [69]. Considering the results of these studies, monitoring oxygen saturation during sedation using pulse oximetry in most patients, regardless of age or the presence of cardiovascular disease, can aid in the early diagnosis and management of desaturation and hypoxemia, thereby helping to prevent hypoxemia.
In the survey, the majority of respondents agreed (strongly agree: 79.7%, agree: 16.1%) that monitoring blood pressure regularly during sedation is helpful for the reduction of cardiovascular complications, including hypotension and hypertension. Maintaining an appropriate blood pressure is crucial for ensuring sufficient perfusion of oxygenated arterial blood into tissues, thereby maintaining circulation. Therefore, regular measurement of blood pressure during sedation and prompt recognition and management of changes in blood pressure can prevent cardiovascular complications.
The majority of respondents to the expert opinion survey agreed (strongly agree: 68.2%, agree: 28.4%) that continuous ECG during sedation in patients with cardiovascular disease was useful for reducing the incidence of cardiovascular complications such as bradycardia, arrhythmia, and cardiac arrest. An observational study investigating the incidence of arrhythmias during sedation in patients with cardiopulmonary disease found that 75% (9/12) experienced arrhythmias such as tachycardia and premature ventricular contraction [68]. In patients with clinically significant cardiovascular disease, timely recognition and management of changes in heart rate, arrhythmias, and myocardial ischemia during sedation via ECG monitoring may help prevent cardiovascular complications.
Most respondents agreed (strongly agree: 65.9%, agree: 28.4%) that maintaining a sedation record is helpful for the effective management of sedation and reduction in the occurrence of medical disputes. Documentation of sedation records allows for appropriate management of patients in the future; it can serve as data for education, research, and statistics; and can be used as a legal document to protect physicians and patients in case of medical disputes.
The results of the internal committee vote on this recommendation was 94.4% and 88.2% for the direction and level of the recommendation, respectively. The user opinion survey results indicated an approval rate of 96.7% for the recommendation direction, and the external expert review showed an approval rating of 60%.
Key Question 11. Does capnography (end-tidal carbon dioxide monitoring added to the standard measurement) during sedation in adult patients reduce the occurrence of hypoxemia?
Recommendation: To reduce the occurrence of hypoxemia during sedation in adult patients, capnography (end-tidal carbon dioxide [ETCO2] monitoring) is recommended.
Recommendation level: Elective use (Do, conditional)
Level of evidence: Moderate
Conditions: If a monitor with ETCO2 monitoring capabilities is available and sensor placement is possible, capnography is recommended during sedation.
Background: Respiratory depression caused by sedation is a relatively common adverse reaction and one of the most critical concerns during sedation. Failure to promptly detect and address inadequate ventilation due to respiratory depression can lead to hypoxemia, resulting in hypoxic tissue damage and potentially progressing to severe outcomes, such as respiratory arrest and cardiac decompensation. Although serious complications are rare, they are irreversible and can be fatal. Therefore, appropriate respiratory status monitoring during sedation is crucial for patient safety. Respiratory monitoring during sedation can be categorized into oxygenation and ventilation. Pulse oximetry, the standard device for monitoring oxygenation, does not directly reflect the ventilation status. If only oxygenation is monitored, ventilation failure may not be promptly detected.
Capnography (i.e., ETCO2 monitoring) provides continuous monitoring of ventilation status and allows for a more direct assessment of ventilation during sedation. The Practice Guidelines for Moderate Procedural Sedation and Analgesia 2018 also recommends capnography during sedation. However, the process used to reach this conclusion is not described in the practice guidelines. Additionally, clinical practice guidelines that reflect the current healthcare situation in South Korea are needed. For this recommendation, the key question was whether capnography (ETCO2 monitoring) contributes to enhanced patient safety during sedation and whether the addition of capnography is associated with a reduction in hypoxemia.
Evidence summary: The selection criteria for the literature search were prospective studies on capnography during sedation, with hypoxemia as an outcome. Twelve randomized studies with groups based on capnography use that compared the incidence of hypoxemia were selected [70–81]. Studies with fewer than 100 patients and those that included pediatric patients were excluded. The criteria for hypoxemia were similar among the selected studies and the incidence data were clearly specified. A total of 4,932 patients were included in the meta-analysis.
The meta-analysis results demonstrated that the use of capnography significantly reduced the risk of hypoxemia (RR: 0.67, 95% CI [0.57, 0.80]); however, the heterogeneity of the results was high (I2 = 65%). The nine randomized studies with an average patient age ≥ 50 years were then analyzed separately. For this analysis, the heterogeneity was not significant (I2 = 19%), and the results showed a significant reduction in the risk of hypoxemia (RR: 0.61, 95% CI [0.55, 0.68]).
When hypoxemia was defined as an oxygen saturation (SpO2) < 90%–95%, capnography led to a significant reduction in hypoxemia in 8 of the 12 included studies. According to the random-effects model, the incidence of hypoxemia was lower (RR: 0.67, 95% CI [0.57, 0.80]). In two studies, hypoxemia occurred more frequently in the group that received capnography monitoring, although no significant difference was observed [73,76]. Among the nine studies that reported the incidence of severe hypoxemia, defined as arterial oxygen saturation (SpO2) < 85%–90%, the risk of severe hypoxemia was lower when capnography was used (RR: 0.62, 95% CI [0.51, 0.75]).
Therefore, capnography is effective at reducing the incidence of severe hypoxemia. The need for increased oxygen supplementation (RR: 0.88, 95% CI [0.75, 1.03]) and assisted ventilation (RR: 0.67, 95% CI [0.30, 1.46]) were reduced, although not significantly.
Although patients may experience discomfort as the sampling line for collecting exhaled gases is placed on the face, it is noninvasive, and there is no risk of clinical harm to the patient. However, capnography may increase healthcare costs [82,83]. The sidestream sampling line is a disposable item, the price of which varies depending on the equipment and product used (generally KRW 10,000–15,000). However, the cost is only reimbursable for general anesthesia under supervision. Water traps, which are recycled consumable products, cost KRW 30,000–50,000 and are generally replaced once or twice a year. However, no disposable equipment is used in the mainstream method, and the sensor costs more than KRW 2,000,000. The cost of the capnography module varies depending on the type of equipment but is approximately KRW 3,700,000, with an average usage period of over 10 years.
However, the value of promptly detecting respiratory depression, which is the most concerning issue in sedation, and mitigating the risk of hypoxemia cannot be solely judged based on costs and should be taken into consideration.
During the first round of voting, 100% of the participating members approved the direction of the recommendation and 93.8% approved the recommendation level. However, while 86.7% of the internal user group familiar with capnography approved the clinical practice guideline, only 60% of the external experts agreed with the guideline, which prompted the committee to present specific indications, as capnography is not required for all sedation procedures (such as endoscopy). After discussions between the committee and individual members, the final recommendation was confirmed under the condition that capnography should be recommended during sedation if a monitor with capnography capabilities is available and sensor placement is possible.
Key Question 12. Does capnography (end-tidal carbon dioxide monitoring added to the standard measurement) reduce the occurrence of hypoxemia during sedation in pediatric patients?
Recommendation: Adding capnography to standard monitoring during sedation of pediatric patients may be considered.
Recommendation level: Elective use (Do, conditional)
Level of evidence: Low
Conditions: Capnography should be used electively when prolonged sedation is expected (e.g., an average expected time > 30 min).
Background: Effective methods of monitoring ventilation vary depending on the age and development of the pediatric patient. Auscultatory and interactive monitoring may be sufficient for most procedures requiring shallow or moderate pediatric sedation. However, early detection of insufficient ventilation during procedures in which the respiratory status of pediatric patients cannot be closely observed (e.g., magnetic resonance imaging) is limited with these traditional methods. In addition, a pulse oximeter cannot be used to monitor a patient’s ventilation state as it will not detect hypoxemia until a few minutes after ventilation failure when supplemental oxygen is administered.
Capnography continuously monitors the state of ventilation and is essential for patients undergoing general anesthesia. However, capnography is used selectively when sedation is performed outside the operating room, depending on the guideline being followed, depth of sedation, and duration of the procedure [1,84,85]. In 2019, the American Academy of Pediatrics and the American Academy of Pediatric Dentistry jointly published a revised edition of their guidelines for pediatric sedation [14]. Currently, continuous capnography is strongly recommended for monitoring ventilation during procedures that require moderate sedation. However, the process used to develop this guideline was not clearly described. The key question for this recommendation is whether capnography during sedation in pediatric patients improves patient safety.
Evidence summary: A literature search and selection process for the main research question resulted in the identification of two randomized controlled trials [86,87]. A total of 254 pediatric patients were included in the meta-analysis: 127 who were monitored using capnography (capnography monitoring group) and 127 who were not (control group). In both studies, the incidences of hypoxemia, c, and interventions to improve ventilation (verbal or physical stimulation, head tilt or chin lift, under-shoulder support, and supplemental oxygen) during sedation were noted.
The benefits were analyzed using data from the two prospective randomized controlled trials. However, no significant difference was noted in the incidence of hypoxemia between the capnography and control (non-capnography) groups in this meta-analysis (RR: 0.52, 95% CI [0.10, 2.56]). Langhan et al. [86] conducted a randomized controlled study that included 154 pediatric patients who underwent various procedures under sedation in the emergency room and found 23 episodes of hypoxemia (oxygen saturation < 95%) among the patients receiving continuous ECG, impedance plethysmography, and pulse oximetry monitoring, and 23 among those additionally receiving capnography, indicating no significant difference between the groups. In contrast, among the 100 patients who underwent upper gastrointestinal endoscopy under sedation who were included in the study conducted by Kılıç and Gerenli [87], two patients in the capnography group and 10 in the control group experienced hypoxemia (oxygen saturation < 90%) (P = 0.0014).
In this meta-analysis, no significant difference was noted in the incidence of hypoventilation between the two groups (RR: 0.94, 95% CI [0.68, 1.29]). In the study by Langhan et al. [86], the proportion of patients who reported hypoventilation increased with increasing sedation time. The change in the ratio of patients who reported hypoventilation per minute increased more significantly in the control group than in the capnography group (change in the rate of hypoventilation per minute: 7.1% vs. 1.0%, P = 0.008, OR: 1.06, 95% CI [1.02, 1.11]). Although a longer sedation time seemed to increase the frequency of hypoventilation, the total incidence of hypoventilation was not dependent on the sedation time, indicating that the use of capnography did not significantly reduce the incidence of hypoventilation.
Regarding airway management interventions performed when oxygenation or ventilation impairment was suspected, the meta-analysis results showed a significant reduction in the frequency of head-tilt or jaw-thrust maneuvers in pediatric patients monitored via capnography (RR: 0.23, 95% CI [0.07, 0.71]). The use of capnography appeared to be beneficial for reducing the frequency of simple airway management interventions (head-tilt and jaw-thrust maneuvers). In the study by Langhan et al. [86], interventions were more likely to result from hypoventilation in the capnography group (OR: 2.26, 95% CI [1.34, 3.81]). Interventions that were not temporally consistent with hypoventilation were associated with a high incidence of hypoxemia (OR: 5.31, 95% CI [2.76, 10.22]). These results suggest that sedation providers reduced the frequency of hypoxemia by performing appropriate airway management interventions once hypoventilation occurred, detected via capnography.
However, as only two studies were included in the analysis and the level of evidence was low resulting from high inconsistency and imprecision, the potential benefit that capnography reduces the frequency of hypoxemia cannot be ruled out. One study reported that capnography reduced the frequency of hypoxemia and increased the frequency of airway management interventions for hypoventilation as sedation time increased, reducing the incidence of hypoxemia [86].
In pediatric patients undergoing moderate sedation, capnography alone cannot reduce the incidence of hypoxemia. With appropriate monitoring of ventilation (observation of chest movements and auscultation of breath sounds) during sedation, most patients can undergo moderate sedation without capnography. However, for patients requiring continuous monitoring of ventilation status (e.g., prolonged procedure time or inability to closely observe the patient), additional monitoring with capnography and timely airway interventions are expected to improve patient safety (Table 7).
In the first round of voting, 100% of the participating members approved the level and direction of the recommendations, with no disagreement regarding the content of this clinical practice guideline. In a user opinion survey of the draft of the clinical practice guideline, 83.3% agreed with the guideline. In the external review, 20% of the respondents strongly agreed, 40% agreed, 0% did not agree or disagree, and 40% disagreed with this clinical practice guideline, indicating that it was generally agreed that capnography should be used.
Key Question 13. Does the assessment of the depth of sedation improve patient safety during pediatric sedation?
Recommendation: The use of a sedation depth assessment tool is recommended for pediatric patients.
Recommendation level: Elective use (Do, conditional)
Level of evidence: Expert consensus/survey
Conditions: If observation and evaluation are not possible during the procedure or examination or if evaluation is difficult because of the patient’s age or developmental status, providers are recommended to monitor the ventilation status and vital signs more closely.
Background: The patient’s level of consciousness should be monitored continuously during sedation to detect any unintentional loss of consciousness or transition to deep sedation. Children have varying abilities to control their behavior during procedures and tests depending on their age and cognitive and emotional development, and infants and children with developmental delays may require deeper sedation. In particular, pediatric patients can show a rapid transition from minimal to deep sedation. In deep sedation, serious adverse effects such as airway obstruction, apnea, and lung aspiration may occur [88,89]. The clinical scales used to assess the depth of sedation include the RSS, the Modified Observer’s Assessment of Alertness/Sedation Scale, and the Pediatric Sedation State Scale. These scales are subjective, and their clinical application may be difficult because the stimuli used for evaluation may interfere with the sedation state. The American Academy of Pediatrics and the American Academy of Pediatric Dentistry have published a series of guidelines regarding pediatric sedation, the 2019 amendments of which recommend ventilation monitoring rather than the use of a specific sedation depth evaluation scale [14]. The 2018 moderate sedation guidelines published in collaboration with the American Society of Anesthesiologists and clinical societies related to sedation recommend the level of consciousness be monitored by measuring the patient’s response to communication during moderate sedation, with no additional descriptions for specific pediatric populations, although insufficient evidence that assessing the patient’s level of consciousness improves patient safety is currently available [1].
Monitoring devices using EEG have been developed as an objective method for assessing sedation depth in adult patients. Although correlations with clinical scales have been suggested for pediatric sedation, these monitoring devices do not provide a reliable distinction between moderate and deep sedation [90–92]. Currently, processed EEG is not recommended for routine clinical practice.
Evidence summary: A literature search was conducted to determine whether the assessment of sedation depth improves patient safety in the pediatric population. However, no suitable studies were identified for the meta-analysis. Recommendations were therefore made based on a review of papers and expert surveys on pediatric sedation, and the level of evidence was thus classified as very low.
A total of 261 user experts responded to the user opinion survey on pediatric sedation. The majority of respondents agreed (strongly agree: 57.1%, agree: 34.9%) that assessing the depth of sedation in pediatric patients is helpful for effective sedation and pain management and reducing complications. To assess sedation depth during pediatric sedation, respondents reported using MOASS (25.3%), EEG-based monitoring (19.9%), and RSS (11.9%). However, the most common response was that only the adequacy of ventilation was monitored (40%).
Both the level and direction of the clinical practice guideline for the selective use of a sedation level assessment tool when performing sedation in pediatric patients were approved by 100% of the participating members. In the user opinion survey, 85% of the respondents agreed with the recommendations, although only 60% agreed with the selective use of a sedation level assessment tool for pediatric sedation in the external expert review of the draft clinical practice guideline.
Key Question 14. Does the recommended standard procedure for airway management (intubation, laryngeal mast airway, etc.) increase patient safety in pediatric patients with dyspnea during sedation?
Recommendation: If respiratory complications, such as dyspnea, occur during sedation in pediatric patients, providers are recommended to follow the appropriate recommendations for airway management.
Recommendation level: Elective use (Do, conditional)
Level of evidence: Expert consensus/survey
Conditions: Elective use in cases where intervention is required for respiratory complications during sedation.
Background: The possibility of patients entering deep sedation or general anesthesia, in which respiratory function is unexpectedly suppressed, is present even during moderate sedation, and appropriate measures can have a considerable impact on patient safety. However, in most sedation practice settings, individual treatment approaches are often adopted according to the clinical experience and judgment of the sedation provider rather than based on specific recommendations on the management of respiratory complications during sedation. Ultimately, the methods of managing complications differ depending on the level of education and training of individual sedation providers, which could significantly affect patient safety. Therefore, this clinical practice guideline was established as a reference for managing respiratory complications in pediatric patients undergoing sedation.
Evidence summary: A literature search of studies on the management of pediatric patients with complications during sedation was conducted. Review articles, treatment guidelines, and studies that included adult patients were excluded. As studies have directly compared the benefits and potential harm associated with management methods in patients with complications during sedation were insufficient, no study was eligible for inclusion in this meta-analysis. This may be attributable to the low incidence rate of respiratory complications during sedation, ethical concerns of intentionally not implementing the recommended management, and challenges in conducting the research. Because no studies were identified that could be used to evaluate the level of evidence for this key question, an expert opinion survey of sedation providers was conducted to develop recommendations for this clinical practice guideline.
Although it was excluded from the meta-analysis during the study selection process, one retrospective observational study was identified that indirectly estimated the effect of differences in managing respiratory complications during pediatric sedation [93]. The study reported that the incidence of respiratory complications during sedation was not significantly correlated with the location of sedation; however, secondary cardiac complications were more likely to occur in primary care settings than in secondary or higher care-based settings. Furthermore, serious harm, such as death and neurological damage, was more common in primary care settings than in secondary or higher care-based settings [93]. Although the specific conditions of each setting were not identified in the study, these results indirectly indicate that differences in the management of respiratory complications during sedation in pediatric patients can significantly affect the development of more serious complications, such as bradycardia and cardiac arrest, as well as patient outcome. Therefore, the majority of respondents to the expert survey reported that guidelines regarding airway management in pediatric patients with dyspnea during sedation would be helpful in ensuring patient safety (strongly agree: 70.5%, agree: 25.3%).
As respiratory complications are a common complication of sedatives, sedation providers should continuously monitor changes in respiratory patterns during the stages of sedation. They should also be familiar with the appropriate treatments and general approaches used in cases of respiratory failure (Table 7). Additionally, they should receive sufficient education and training regarding treatment methods (including head tilt, jaw thrust, under-shoulder support, oral [nasal] airway, and bag-mask ventilation) that can be used during the early stages of respiratory complications. In addition, sedation providers should understand that it is possible to insert a supraglottic airway and perform tracheal intubation if respiratory failure progresses further, and appropriate devices should be prepared prior to sedation. According to the expert survey, the most important methods used to treat dyspnea during sedation in pediatric patients are as follows: head tilt or jaw thrust (86.2%), bag-mask ventilation (76.6%), oral (nasal) airway (71.6%), under-shoulder support (20.7%), and supraglottic airway device (18.4%).
For this clinical practice guideline on the management of respiratory complications during sedation in pediatric patients according to appropriate standards, 94.4% of the clinical practice guideline committee agreed on the direction and level of recommendation. After consultation and review by external experts, 100% agreement with the recommendations was obtained, and the user opinion survey showed 99.2% agreement. Therefore, this clinical practice guideline was finalized by reflecting on the results of voting by the clinical practice guideline committee, external expert consultation, and user consultation and voting.
Key Question 15. Are well-defined discharge criteria essential for safely discharging patients after sedation?
Recommendation: Providers are recommended to apply appropriate discharge criteria when discharging adult patients who have undergone sedation after recovery.
Recommendation level: General use (Do, strong)
Level of evidence: Expert consensus/survey
Background: It is important to decrease the risk of sedation-related complications and ensure patient safety not only during but also after the sedation procedure. To reduce complications related to sedation and maintain patient safety after sedation, patients should be assessed using well-established discharge criteria [94].
Evidence summary: A literature search was conducted for studies assessing discharge criteria after sedation in adult patients. Review articles, treatment guidelines, retrospective studies, and studies with fewer than 100 participants or pediatric patients were excluded. As there were insufficient studies that directly compared the benefits and harms of patient evaluation based on discharge criteria and the reduction in complications, expert opinion surveys of sedation providers were collected. The recommendations were based on the results of the survey; hence, the level of evidence was considered very low.
Patient assessment using appropriate discharge criteria is believed to improve patient safety after sedation and reduce sedation-related complications. Most respondents to the expert survey reported that the Modified Aldrete Score was a suitable scoring system and assessment tool for determining a patient’s preparedness for discharge after sedation. Most respondents also agreed that assessing patients in a recovery area for 30 min after sedation while recording their level of consciousness and vital signs at regular intervals reduces the occurrence of complications after sedation. Furthermore, providing education on water and food intake, emergency contact information, driving restriction instructions, and discharge with a caregiver can help reduce the occurrence of complications after sedation.
Discharge criteria focused on enhancing patient stability and reducing potential complications following sedation, such as postoperative nausea and pain, should be selected and presented in a simple manner (Table 8).
All participating members expressed 100% agreement with this recommendation, and no objections were expressed. However, due to the variety of possible procedures and sedation methods, several members expressed that using a single set of discharge criteria is inadequate and the sedation provider’s judgment is important. External experts agreed with the recommendations (strongly agree: 20%, agree: 60%, disagree: 20%). Nevertheless, in the user-opinion survey, a majority vote of 98.3% was obtained. After discussions with the committee and individual members, a consensus was reached that the benefits outweighed the potential harm, and the original draft of the clinical practice guideline was finalized.
Conclusion
The present work, the Korean clinical practice guidelines for diagnostic and procedural sedation, is developed as a clinical practice guideline in accordance with the clinical situation related to sedation in Korea. The development method was "de novo", and 15 key-questions were selected based on the opinions of various experts, and 15 recommendations were made. The purpose of these evidence-based multidisciplinary clinical practice guidelines is to ensure the safety and efficacy of sedation, thereby contributing to patient safety and ultimately improving public health. Although proposed guidelines are not mandatory, we hope to assist sedation providers and patients in sedation-related decision making for diagnostic and therapeutic examinations or procedures.
Notes
Funding
This work was supported by the Korean Society of Anesthesiologists.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Sang-Hyun Kim (Conceptualization; Data curation; Project administration; Resources; Supervision; Validation; Writing – original draft; Writing – review & editing)
Young-Jin Moon (Data curation; Investigation; Methodology; Writing – original draft; Writing – review & editing)
Min Suk Chae (Investigation; Methodology; Writing – original draft)
Yea-Ji Lee (Investigation; Methodology; Writing – original draft)
Myong-Hwan Karm (Investigation; Methodology; Writing – original draft)
Eun-Young Joo (Investigation; Methodology; Writing – original draft)
Jeong-Jin Min (Formal analysis; Investigation; Writing – original draft)
Bon-Nyeo Koo (Investigation; Methodology; Project administration; Supervision; Writing – original draft)
Choi Jeong-Hyun (Data curation; Investigation; Methodology; Writing – original draft; Writing – review & editing)
Jin-Young Hwang (Data curation; Investigation; Writing – original draft)
Yeonmi Yang (Formal analysis; Investigation; Writing – original draft)
Min A Kwon (Data curation; Investigation; Methodology; Writing – original draft)
Hyun Jung Koh (Investigation; Methodology; Writing – original draft)
Jong Yeop Kim (Formal analysis; Investigation; Writing – original draft)
Sun Young Park (Data curation; Formal analysis; Writing – original draft)
Hyunjee Kim (Investigation; Methodology; Writing – original draft)
Yang-Hoon Chung (Formal analysis; Investigation; Writing – original draft)
Na Young Kim (Investigation; Methodology; Writing – original draft)
Sung Uk Choi (Formal analysis; Investigation; Supervision; Validation; Writing – original draft)