Use of oxygen reserve index during bronchoscopic balloon dilation for subglottic stenosis in a patient with left ventricular assist device implantation -a case report-
Article information
Abstract
Background
Monitoring the oxygenation status is crucial during general anesthesia to ensure patient safety. Although noninvasive pulse oximetry is commonly used to monitor percutaneous oxygen saturation (SpO2), it may not accurately reflect changes in oxygen partial pressure when the latter is excessively high or low. The oxygen reserve index (ORi) provides real-time information about the oxygen reserve status.
Case
We present a case of successful management of subglottic stenosis using balloon bronchoscopy in an infant with a left ventricular assist device implantation under ORi monitoring to predict hypoxemia during the surgical procedure.
Conclusions
Utilizing ORi monitoring during anesthesia for procedures involving apnea in critically ill infants can help predict impending desaturation before a drop in SpO2 occurs, allowing anesthesiologists to effectively anticipate and manage the apnea period. Continuous ORi monitoring offers valuable insights during surgical procedures, especially in infants with compromised respiratory and cardiovascular functions.
Monitoring oxygenation status during general anesthesia is essential for respiratory management. Most anesthesiologists use noninvasive pulse oximetry to monitor percutaneous oxygen saturation (SpO2) to detect hypoxemia and adjust the inspired oxygen concentration without invasive tests such as arterial blood gas analysis [1]. However, in cases where the actual oxygen partial pressure is excessively high or low, the SpO2 reading can still appear close to 100%. Once the SpO2 reading begins to decrease, it indicates a significant drop in the oxygen partial pressure, and the rate of decrease in SpO2 accelerates rapidly, increasing the risk of hypoxemia [2,3]. The oxygen reserve index (ORi) (Masimo Corp.) is a time indicator of oxygen reserves. It not only displays oxygen saturation, but also indicates the oxygen reserve status on a scale of 0.00 (no reserve) to 1.00 (much reserve) that is different from noninvasive pulse oximetry [4].
We report a case of successful management of subglottic stenosis using balloon bronchoscopy in an infant with left ventricular assist device (LVAD) implantation under ORi monitoring to predict hypoxemia during the surgical procedure.
Case Report
This study was approved by the Institutional Review Board of Pusan National University Yangsan Hospital (IRB No. 05-2022-215), and written informed consent for publication was obtained from the parents as the patient was a minor. A male infant was diagnosed with dilated cardiomyopathy at two months of age and underwent implantation of a LVAD (Berlin EXCOR ®, 10 ml; Berlin Heart AG). Following extubation at three months of age, the patient developed mild hoarseness and later presented with nasal flaring, chest retraction, and stridor. Reintubation was performed using an uncuffed endotracheal tube (ETT) with an internal diameter (ID) of 3.5 mm for two days. Beta-agonist inhalation therapy was initiated; however, the patient’s condition progressed to grade 3 stenosis (Fig. 1). Tracheostomy was considered, but the parents objected. Alternatively, bronchoscopic balloon dilation was planned.
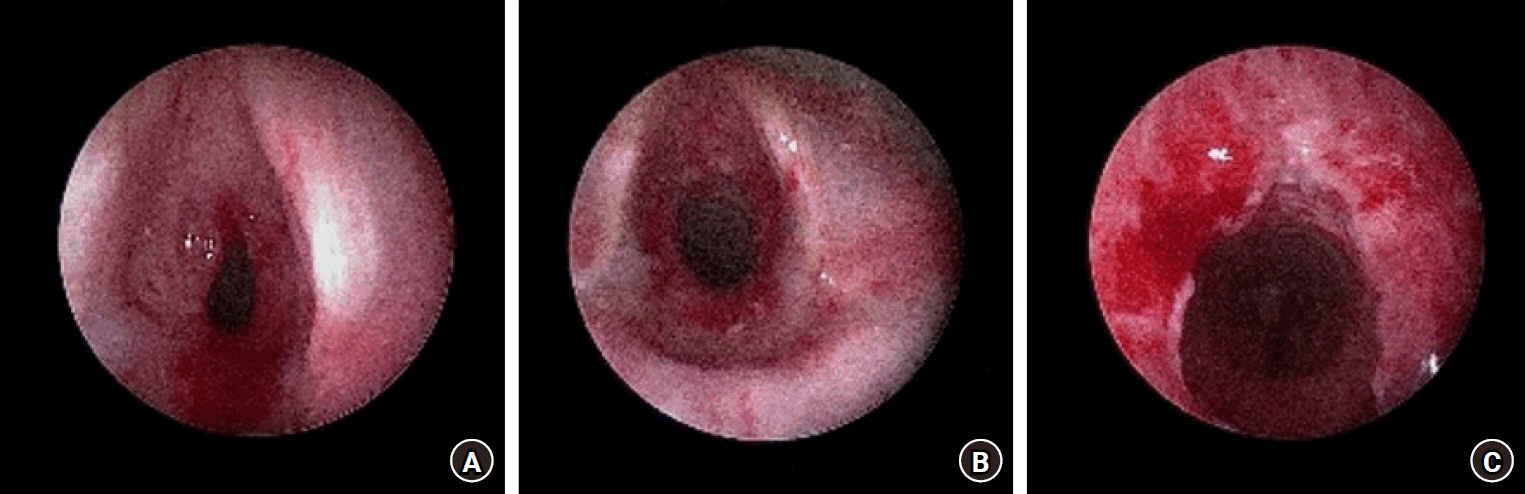
Bronchoscopic view of subglottic lesion. (A) Preoperative diagnosis is grade 3 subglottic stenosis. (B, C) The lesion improves to grade 1 subglottic stenosis after bronchoscopic balloon dilation.
At the time of admission to the operating room, the patient was five months old, with a length of 57 cm and weight of 5.9 kg. SpO2 was maintained at 100% with 1 L/min of oxygen via a nasal cannula. The blood pressure was 119/63 mmHg and the heart rate was 172 beats/min. To monitor the risk of rapid desaturation during surgery, a sensor (rainbow® sensor, R2–25, Revision L, Masimo Corp.) for measuring ORi was attached to the patient’s right big toe. ORi was monitored using the own system (Root® with Radical-7® system; Masimo Corp.). During anesthesia induction, oxygen was continuously supplied at a rate of 5 L/min through a nasal cannula, and an additional 5 L/min was administered using a mask. An intravenous injection with 8 mg of ketamine, 4 mg of rocuronium, and 10 μg of fentanyl was administered. The procedure was anticipated to be difficult because of involuntary movement caused by surgical stimulation; therefore, a neuromuscular blocker was administered despite predicting the occurrence of apnea. Subsequently, a laryngeal mask airway (LMA) #1 (i-gel®; Intersurgical Ltd.) was inserted. Anesthesia was maintained with an infusion of 4–12 mg/kg/h of 1% propofol, and the bispectral index was maintained at 33–37.
After achieving SpO2 of 100% and ORi of 1, LMA was removed and a suspension laryngoscope was inserted into the glottis by the surgeon. Bronchoscopic balloon dilation was started when ORi reached 1, and continued for approximately 30 s, even after ORi reached 0. Apneic oxygenation was performed using oxygen supplied at a rate of 5 L/min through the nasal cannula during the procedure. Mechanical ventilation via ETTs with IDs of 5.0 and 6.0 mm was also attempted, but resulted in near apnea. The total time for the first procedural attempt was approximately 3–4 min, with 3 min from ORi 1 to 0 and 30 s from ORi 0 to SpO2 80% (Fig. 2A). The apnea period was extended to ensure sufficient procedural time, even after the ORi reached 0. As a result, despite initiating ventilation with 100% oxygen when SpO2 was 80%, there was a temporary drop in SpO2 by 40% to 60% after approximately 30 s. Fortunately, there was no hemodynamic instability, such as bradycardia or hypotension. When the ORi reached 0, the LMA was reinserted and rescue ventilation was performed with 100% oxygen. It took approximately 1 min to reach SpO2 above 97% and approximately 3 min for ORi to change from 0 to 1. The procedure was performed four times, with each trial lasting for 1 min 30 s at pressures of 760, 1140, 1520, and 1900 mmHg, respectively. Each trial also had a temporary drop in SpO2 by 40% to 60% similar to the first attempt (Fig. 2B) although there was no hemodynamic instability.
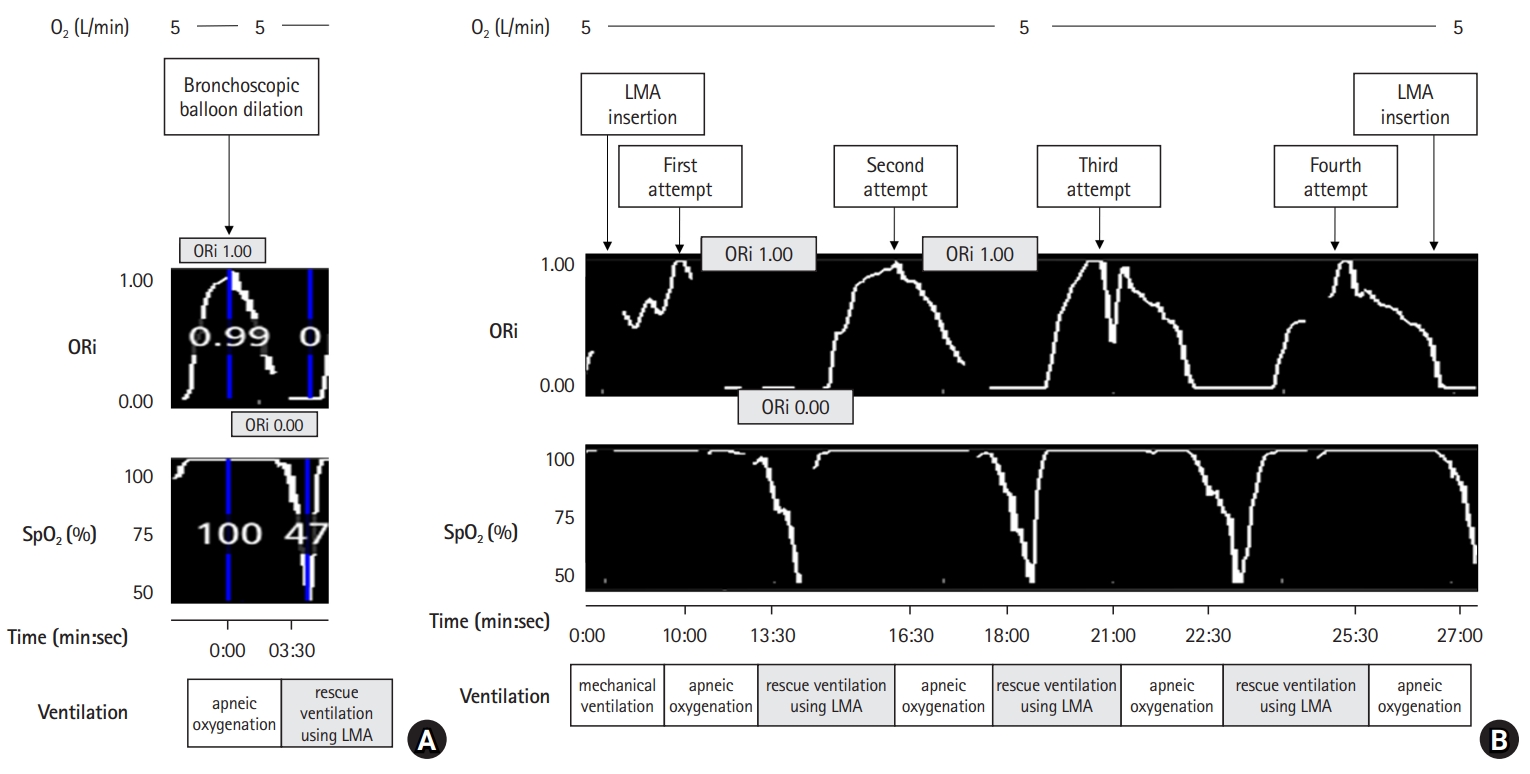
Change of ORi, and SpO2 during bronchoscopic balloon dilation. (A) The total duration for the first procedural attempt is approximately 3–4 min, with 3 min from ORi 1 to 0 and another 30 s from ORi 0% to 80% SpO2. (B) The procedure is performed four times, with each trial lasting 1 min 30 s, at pressures of 760, 1140, 1520, and 1900 mmHg, respectively. It took approximately 3 min for ORi to change from 1 to 0. The apnea period has been extended to ensure sufficient procedural time, even after the ORi reached 0. As a result, despite initiating ventilation with 100% oxygen when the SpO2 was 80%, there is a temporary drop in SpO2 by 40% to 60% after approximately 30 s. LMA: laryngeal mask airway, O2: oxygen, ORi: oxygen reserve index, SpO2: percutaneous oxygen saturation.
The lesion improved to grade 1 subglottic stenosis (Fig. 1), and the procedure was successfully completed. The infusion of propofol was discontinued, and sugammadex (Bridion®; MSD) 20 mg was administered to reverse the neuromuscular block because the “train of four” count was 0 after the end of the surgery, and about 4 mg/kg of sugammadex was considered reasonable. It was confirmed that the “train of four” ratio was > 90%. LMA was subsequently removed after smooth, spontaneous breathing was confirmed. The patient was shifted to the ward under the supervision of an attending pediatrician.
Discussion
In anesthesia for procedures that cause apnea, the ability to predict impending desaturation before a drop in oxygen saturation occurs is crucial for ensuring patient safety, especially in vulnerable populations such as neonates and infants with poor respiratory and cardiovascular function. Such patients have limited physiological reserves and are susceptible to oxygenation issues.
Neonates and infants have distinct characteristics in their respiratory and cardiovascular systems that make them vulnerable to oxygenation problems; their airways are anatomically different, with a narrow upper airway and relatively large epiglottis. Additionally, their laryngeal cartilages are not fully developed, leading to frequent obstructions during inspiration. Newborns and infants have narrower airways than adults, making them more prone to ventilation and diffusion impairments. Moreover, they have a lower functional residual capacity, low oxygen reserve, poor tolerance to apnea, and susceptibility toward hypoxemia and atelectasis. Furthermore, apnea in these populations, especially in children with reduced cardiopulmonary function, can rapidly lead to desaturation and systemic circulatory crises [5].
Once the partial pressure of arterial oxygen (PaO2) reaches 80 mmHg or higher, SpO2 tends to remain close to 100%. Beyond this point, further increases in PaO2 do not result in an increase in SpO2, as the hemoglobin is already fully saturated with oxygen. Consequently, SpO2 cannot reliably predict PaO2 or reflect the oxygen reserves after complete oxygenation of hemoglobin [6]. When SpO2 starts to decrease, it indicates that PaO2 has undergone a steep decline in the oxygen–hemoglobin dissociation curve. Therefore, even if immediate actions are taken to improve oxygenation when SpO2 begins to drop, rapid decrease in PaO2 can lead to a sharp decline in SpO2 and hypoxemia [2]. Although arterial blood gas analysis provides an accurate measurement of PaO2, its invasive and intermittent nature limits its ability to monitor sustained hypoxemia continuously [7].
ORi is a novel noninvasive parameter that reflects real-time oxygen reserves. It is represented as a value between 0 and 1, where 0 represents no oxygen reserve and 1 represents maximum oxygen reserve. ORi reflects PaO2 values in the range of 100 to < 200 mmHg [8,9]. Previous studies in adult and pediatric patients have shown that ORi decreases approximately 30 s before the onset of SpO2 decline, providing sufficient time for interventions to prevent hypoxemia [6,10]. A study by Szmuk et al. [6] focused on changes in ORi and SpO2 in 25 relatively healthy pediatric patients after performing preoxygenation and endotracheal intubation, and disconnecting the anesthesia circuit from the ETT. This may not be directly applicable to vulnerable patients with compromised respiratory and cardiovascular functions because relatively healthy pediatric patients maintained SpO2 levels during apnea periods. However, continuous monitoring of changes in ORi can help determine whether a patient has a full oxygen reserve. By observing the depletion of the oxygen reserve and the start of a decrease in SpO2, it is possible to predict hypoxia during the procedure, and anesthesiologists can provide information on when to stop the procedure. ORi increases from 0.0 to 1.0 even after SpO2 reaches 100%, indicating that the capacity of the oxygen reserve has reached its maximum value that can present the optimal timing for procedures such as balloon bronchoscopy to ensure patient safety. However, there was a time gap between balloon bronchoscopy and the initiation of ventilation in the current case, prompting reoxygenation after ORi reached 0. This resulted in a temporary but significant decrease in SpO2 to levels as low as 45%. From this point of view, it was somewhat disappointing not to apply a transnasal humidified rapid-insufflation ventilatory exchange using a high-flow nasal cannula.
ORi has a significant correlation with PaO2 values in the range of 100 to < 200 mmHg. When PaO2 is > 100 mmHg, the ORi value is ≥ 0.24, and when PaO2 is > 150 mmHg, the ORi value is ≥ 0.55 [9]. If the balloon bronchoscopic procedure had been interrupted and reoxygenation initiated when ORi fell between 0.24 and 0.55, in addition to the ORi alarm of downward trends, it could have prevented the infant from experiencing further desaturation.
In premature neonates, infants, and children, apnea induced by anesthesia or airway-related surgical procedures can lead to rapid desaturation because they have lower oxygen reserves and consume more oxygen than adults [5]. In infants with poor respiratory and cardiovascular functions, the severity of desaturation is even more pronounced and can potentially result in bradycardia and cardiac arrest [11,12]. By utilizing ORi, it is possible to predict expected desaturation during procedures involving apnea in critically ill infants, allowing anesthesiologists to anticipate and manage the apnea period effectively. This helps protect patients from severe hypoxemia. Additionally, real-time estimation of PaO2 can be achieved using ORi that is beneficial not only in cases of decreased PaO2 but also in critically ill neonates and infants to prevent complications such as absorption atelectasis, pulmonary injury, and retinal injury caused by excessively high PaO2 levels [13,14].
Notes
Funding
This work was supported by a 2023 research grant from Pusan National University Yangsan Hospital.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Author Contributions
Jimin Lee (Conceptualization; Data curation; Investigation; Methodology; Validation; Visualization; Writing – original draft; Writing – review & editing)
Minwoo Chung (Writing – review & editing)
Eui-Suk Sung (Writing – review & editing)
Jung-Pil Yoon (Writing – review & editing)
Yeong Min Yoo (Writing – review & editing)
Jaesang Bae (Writing – review & editing)
Hee Young Kim (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Supervision; Writing – original draft; Writing – review & editing)
