Anesthetic management with remimazolam for laryngectomy in a severely underweight patient with facioscapulohumeral muscular dystrophy
Article information
Facioscapulohumeral muscular dystrophy (FSHD) is the third most common muscular dystrophy worldwide [1]. It is characterized by the slowly progressive wasting of facial, scapular, and humeral muscles in adolescence but eventually extends to the lower extremities [1]. As with other muscular dystrophies, total intravenous anesthesia (TIVA) is the preferred. Because volatile anesthetics can be associated with anesthesia-induced rhabdomyolysis, hyperkalemia, and cardiac arrest [2]. We report the successful administration of remimazolam TIVA in a severely underweight patient with FSHD. This study was approved by the Institutional Review Board of Yeouido St. Mary’s Hospital (approval number: SC23ZESI0019). The patient authorized the publication of this letter with anonymized details.
A 27-year-old female patient (height: 165 cm, weight: 30 kg, body mass index [BMI]: 11.02 kg/m2) was scheduled for a total laryngectomy. She was diagnosed with FSHD 17 years earlier. The disease con tinued to progress, and she finally lost her ability to ambulate. She underwent a tracheotomy one year earlier and was using a home ventilator. She had frequent aspiration pneumonia, so a total laryngectomy was decided.
Her initial vital signs were within the normal range in the operating room. Neuromuscular blockade was monitored via train-of-four (TOF) acceleromyography on the right hand for the ulnar nerve and the left foot for the sural nerve simultaneously. After the tracheotomy tube was connected to the circuit of the anesthesia workstation, remimazolam was infused at a rate of 12 mg/kg/h with remifentanil (0.3 μg/kg/min) under bispectral index (BIS) monitoring. After approximately 10 mg of remimazolam was infused, her BIS value decreased to below 70, and the eyelash reflex disappeared. Then, 20 mg of rocuronium was injected, and the remimazolam infusion rate was decreased to 2 mg/kg/h. The TOF ratio was initially 100% on both sites, and the TOF count became 0 on the sural nerve after about 80 s but remained at 1 even after about 90 s on the ulnar nerve, so an additional 10 mg of rocuronium was administered. After we confirmed the disappearance of the TOF count on the ulnar nerve after 60 s and BIS value less than 60, we changed the tracheotomy tube to an armored 6.5 mm endotracheal tube. The left dorsalis pedis artery was cannulated and connected to a FloTracTM/Vigileo system (Edwards Lifesciences, USA) to measure cardiac output.
For maintenance, we continued the infusion of remimazolam at a rate of 1.5–2 mg/kg/h and remifentanil at a rate of 0.2–0.45 μg/kg/min to achieve a BIS of 40–60. Stable hemodynamic variables were maintained (Fig. 1), with a cardiac index value of 3.5–4.7 L/min/m2 throughout the whole procedure. Additional rocuronium at 10 mg was administered 3 h after anesthesia induction because twitches raised to 4/4 in the TOF count on both sites. Total laryngectomy was finished after 3.5 h. We discontinued remimazolam and remifentanil infusion and administered 60 mg of sugammadex. We confirmed that the patient opened her eyes upon verbal command about 10 min later.
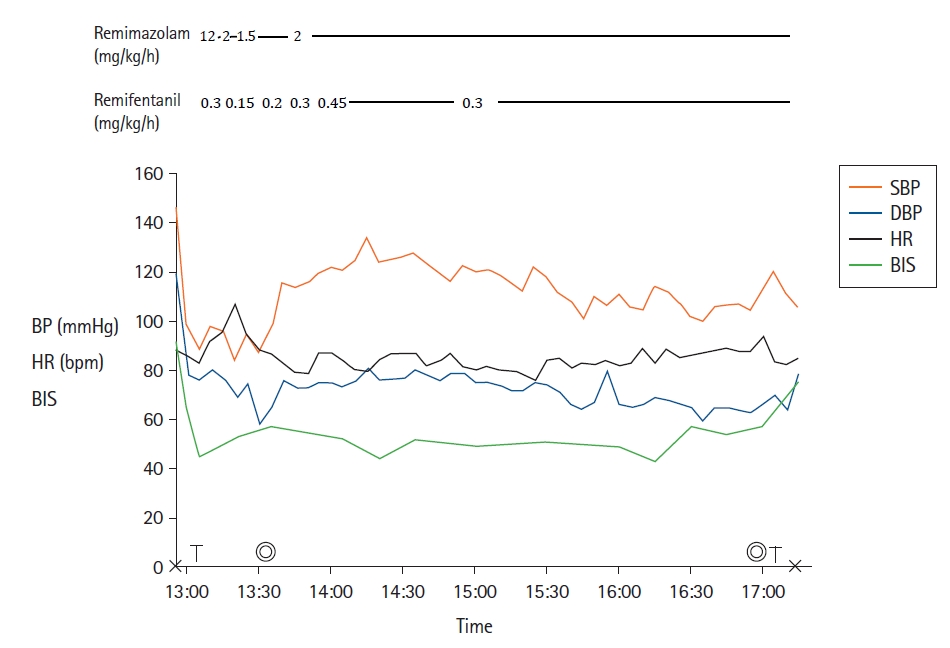
Anesthesia record. “X” and the double circles represent the start/end of anesthesia and surgery, respectively. “T” represents tracheal tube change. BP: blood pressure, bpm: beats per minute, DBP: diastolic blood pressure, HR: heart rate, SBP: systolic blood pressure, BIS: bispectral index.
Severely underweight patients with a BMI of < 13 kg/m2 are known to be prone to critical perioperative cardiopulmonary complications [3]. We chose remimazolam TIVA because propofol can reduce cardiac contractility and blood pressure. Changes in the cardiac index during anesthesia were within the normal range (> 3.5 L/min/m2). This may have been because she was young and cardiac function was well-maintained at an adequate depth of anesthesia with remimazolam.
Remimazolam is suitable for TIVA because it has better hemodynamic stability than propofol and ensures rapid recovery due to the presence of an antagonist [4]. However, adequate doses of remimazolam for severely underweight patients are not yet known. In underweight patients, the dose titration of anesthetics based on total body weight (TBW) can result in underdosing and an inadequate depth of anesthesia. Population pharmacokinetic modeling revealed that adjusted body weight (ABW) was best fitted to predicting plasma concentrations of remimazolam [5]. The TBW of the patient was 30 kg, and the ABW was approximately 59.9 kg. The patient lost consciousness after the administration of 10 mg of remimazolam that was about 0.17 mg/kg of ABW. It was less than the 0.25–0.33 mg/kg range suggested in a previous study as the optimal induction dose for patients younger than 40 years [6]. We administered remimazolam at a rate of 1 mg/kg/h for the ABW (2 mg/kg/h for TBW) during the maintenance of anesthesia. Pharmacokinetic analysis showed that female patients needed a 10% higher injection rate than male patients to maintain remimazolam concentrations, and American society of anesthesiologists physical status (ASA-PS) III/IV patients needed a 20% lower infusion rate than ASA-PS I/II patients [5]. Although the patient’s ASA-PS was IV, the anesthetic requirement was not reduced when the dose was based on ABW. That may have been because this patient was young, female, and the procedure was painful. The anesthesia for this patient was maintained deeply at a BIS of < 50 at this infusion rate. Although we maintained deep anesthesia for about 3 h, the emergence time was not affected. It was comparable to a previous report where the time to awareness from the discontinuation of remimazolam was approximately 8–9 min in ASA-PS III patients who un derwent over 3 h of surgery [4].
The TOF ratio was initially higher in the sural nerve than in the ulnar nerve, but the TOF count disappeared more rapidly in the sural nerve. Although there was muscle atrophy and discrepancy in the TOF counts during induction, the recovery from neuromuscular blockade was similar on both sites. The sensitivity to rocuronium was similar to that of normal subjects.
In conclusion, remimazolam dose titration using the ABW was shown to be safe for general anesthesia under the monitoring of BIS and hemodynamic variables in an underweight patient with FSHD. It may be helpful to monitor neuromuscular blockade in both the upper and lower extremities.
Notes
Funding: None.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
Author Contributions: Kwon Hui Seo (Conceptualization; Data curation; Investigation; Writing – original draft; Writing – review & editing); Ji Yung Lee (Conceptualization; Data curation; Methodology; Writing – review & editing)
