Comparison of the effects of open and closed aspiration on end-expiratory lung volume in acute respiratory distress syndrome
Article information
Abstract
Background
Alveoli tend to collapse in patients with acute respiratory distress syndrome (ARDS). Endotracheal aspiration may increase alveolar collapse due to the loss of end-expiratory lung volume (EELV). We aimed to compare the loss of EELV after open and closed suction in patients with ARDS.
Methods
This randomized crossover study included 20 patients receiving invasive mechanical ventilation for ARDS. Open and closed suction were applied in a random order. Lung impedance was measured using electric impedance tomography. The change in end-expiratory lung impedance (EELI) end of suction and at 1, 10, 20, and 30 min after suction, was used to represent the change in EELV. Arterial blood gas analyses and ventilatory parameters such as the plateau pressure (Pplat), driving pressure (Pdrive), and compliance of the respiratory system (CRS) were also recorded.
Results
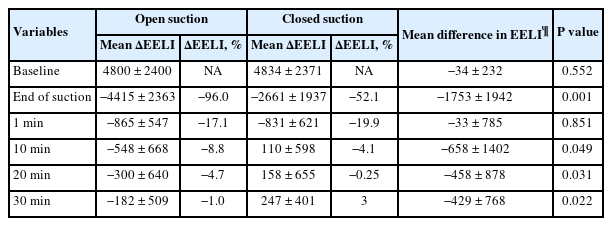
Less volume loss was noted after closed suction than after open suction (mean ΔEELI: −2661 ± 1937 vs. −4415 ± 2363; mean difference: −1753, 95% CI [−2662, −844], P = 0.001). EELI returned to baseline 10 min after closed suction but did not return to baseline even 30 min after open suction. After closed suction, the Pplat and Pdrive decreased while the CRS increased. Conversely, the Pplat and Pdrive increased while the CRS decreased after open suction.
Conclusions
Endotracheal aspiration may result in alveolar collapse due to loss of EELV. Given that closed suction is associated with less volume loss at end-expiration without worsening ventilatory parameters, it should be chosen over open suction in patients with ARDS.
Introduction
The purpose of mechanical ventilation is to provide adequate gas exchange while preventing lung injury. Alveolar collapse at end-expiration is a common phenomenon in various respiratory conditions, including acute respiratory stress syndrome (ARDS) [1]. Adequate positive end-expiratory pressure (PEEP) is crucial for preventing alveolar collapse and atelectotrauma.
As intubated patients are unable to clear secretions from the airways spontaneously, the airways must be cleaned periodically. Endotracheal aspiration, which is one of the most common procedures performed in the intensive care unit (ICU), clears the respiratory tract, prevents atelectasis, and improves oxygenation [2]. Although endotracheal aspiration is essential for intubated patients, it can have harmful effects such as oxygen desaturation and alveolar derecruitment [3,4].
Endotracheal aspiration can be performed either using open suction, in which the patient is disconnected from the ventilator, or closed suction, in which a sterile catheter is inserted into the ventilator circuit without disconnection. Under open suction, an abrupt drop in airway pressure due to disconnection from the ventilator and negative pressure aspiration may cause alveolar derecruitment and collapse. Because the patient remains connected to the ventilator in closed aspiration, this technique protects against alveolar derecruitment and results in less volume loss [5]. Heinze et al. [6] showed that the functional residual capacity (FRC) is reduced after endotracheal suctioning, regardless of whether closed or open suction is used, and remains low 20 min after aspiration. Although end-expiratory lung volume (EELV) loss is lower in closed suction than in open suction, slower recovery has been observed when closed suction is used in post-cardiac surgery patients [7].
EELV can be easily and accurately measured at the bedside using electric impedance tomography (EIT). The basic principle of electric impedance is based on alternating current injection and voltage measurements using surface electrodes placed around the chest wall. The electrical properties of the chest change with inspiration and expiration owing to variations in air content, and changes in impedance resulting from ventilation can be measured using an electric impedance device [8]. These changes in impedance represent changes in EELV because of the strong linear relationship between impedance and EELV [9].
Studies examining the effect of the endotracheal aspiration method on EELV have been conducted in surgical patients [6,7]; however, the effect is unclear in cases such as ARDS where the alveoli are more prone to collapse. This study compared the effects of the endotracheal aspiration method on EELV in patients with ARDS.
Materials and Methods
This randomized crossover study was conducted between September 15 and October 30, 2022, at the ICU of the University of Health Sciences Turkey, Dr. Suat Seren Chest Disease and Surgery Training and Research Hospital, which is a tertiary hospital specializing in pulmonary diseases. Our study was approved by the Ethics Committee of the University of Health Sciences, Dr. Suat Seren Chest Disease and Thoracic Surgery Teaching and Research Hospital (IRB number: 2022/2-7). Written informed consent was obtained from all participants or their next of kin. The study was registered at ClinicalTrials.gov under the number NCT05537974 and was conducted in accordance with the Declaration of Helsinki, 2013.
Participants
Patients aged ≥ 18 years who underwent invasive mechanical ventilation due to ARDS were included in the study. ARDS was diagnosed according to the Berlin criteria [10]. Patients were excluded from the study if they were hemodynamically unstable (systolic blood pressure < 90 mmHg or mean arterial pressure < 60 mmHg), had an air-leak condition such as pneumothorax, had high FiO2 levels (> 60%), or had a cardiac pacemaker.
Study protocol
The suction procedures were performed in a random order with a 60-min washout period between. Patients were randomized after intubation using sealed envelopes. A flowchart of the study is shown in Fig. 1.
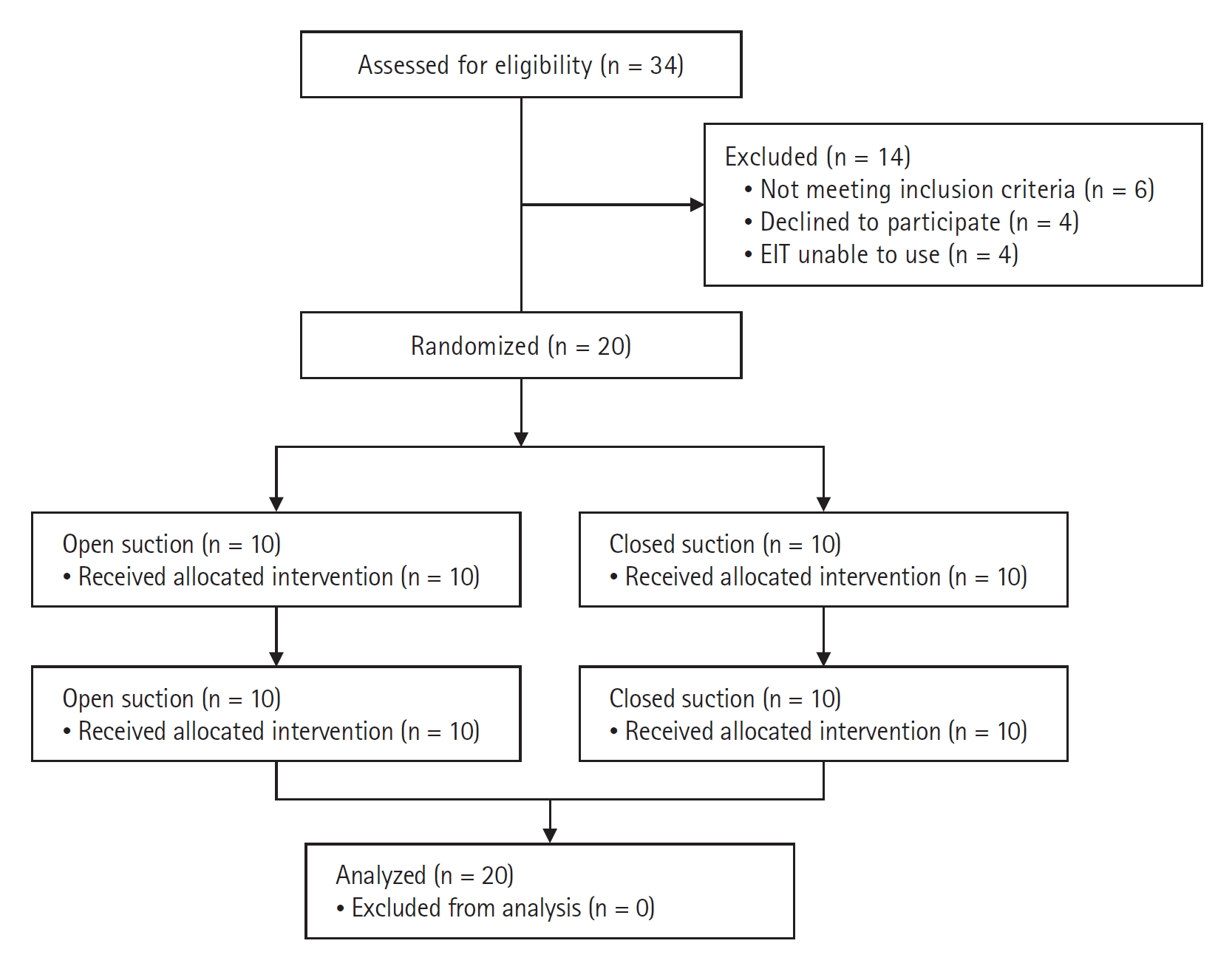
Consolidated Standards of Reporting Trials (CONSORT) flowchart of study selection. EIT: electrical impedance tomography.
An electrical impedance tomography belt (PulmoVista® 500, Dräger Medical GmbH) with 16 electrodes was placed around each patient’s chest between the fifth or sixth intercostal space. All patients were intubated with an 8.0-mm or 8.5-mm diameter endotracheal tube and mechanically ventilated (Galileo GOLD; Hamilton Medical AG) using a lung-protective ventilation strategy in continuous volume mandatory ventilation mode. The tidal volume (Vt) was set at 4–8 ml/kg, plateau pressure (Pplat) at < 30 cmH2O, and FiO2 was titrated to maintain a SaO2 of 88%–92%. The PEEP level was set by an intensivist who was blinded to the study.
All suctioning was performed within the first 24 h after intubation. Before suctioning, patients were ventilated with 100% oxygen for 60 s. The negative aspiration pressure was set at 150 mmHg [4]. A 14 F suction catheter was used for both open (Bıçakçılar Medical Equipment) and closed (Shaoxing Reborn Medical Device) suctioning. The patient was disconnected from the ventilator for open suction but not for closed suction. For both open and closed suction, the aspiration catheter was advanced until resistance was met and was then withdrawn 1 cm before aspiration. During endotracheal suctioning, negative pressure was applied twice for 5 s. All suction maneuvers were performed by the intensivists.
Measurements
End expiratory lung impedance (EELI) was measured using EIT at baseline (1 min before suctioning) and at 1, 10, 20, and 30 min after suctioning. Changes in EELI were used to represent changes in EELV, as a strong linear relationship between lung impedance and volume was established by Hinz et al. [9]. In that study, the EELV was measured using the open-circuit nitrogen washout maneuver, and an increase in the EELV was induced by a stepwise increase in the PEEP. A linear relationship between the increase in EELV and changes in EELI were seen (R2 = 0.95) [9].
Oxygen saturation was measured via pulse oximetry, and pulse and arterial blood pressures were recorded. Arterial blood gas levels were measured before and 30 min after suctioning. Mechanical ventilatory parameters, such as the Vt, Pplat, PEEP, FiO2, driving pressure (Pdrive), and static compliance of the respiratory system (CRS), were also recorded. All measurements were performed under passive conditions (patients were sedated and ventilation was thus not triggered). The ventilatory settings were maintained constant before, during, and after the suction maneuvers were performed.
Statistical analysis
Continuous variables are represented as the mean ± SD if they are normally disturbed and as the median (Q1, Q3) if they are not normally disturbed. The change in EELI compared to baseline was used to represent the change in EELV. The differences between the baseline and post-suction values (ΔEELI values) in open and closed suction were compared using paired t-tests because of the normal distribution of the data. Differences in the respiratory variables (Pplat, Pdrive, etc.) were compared using the Wilcoxon signed-rank test because the data were not normally distributed. Statistical significance was set at P < 0.05. The sample size was based on a 20% difference in the reduction in EELI between the groups. Twenty patients were thus required to determine this difference with 80% power at a 5% significance level. It was assumed that the paired difference in the EELI (open minus closed) had a normal distribution, with an SD of 30%.
Results
Twenty patients with ARDS were included in the study. Thirteen (65%) of the participants were male, and the mean age was 64.2 ± 14.1 years. All patients had ARDS due to pneumonia (60% bacterial and 40% viral). The clinical features and baseline characteristics of the participants are summarized in Table 1. All measurements were successfully recorded for all patients, and there were no missing data.
The baseline electrical impedance values were comparable between open and closed suction. More volume loss after suctioning was found with open suction than with closed suction. The mean ∆EELI was −4415 ± 2363 impedance units in open suction and −2661 ± 1937 impedance units in closed suction (mean difference: −1753, 95% CI [−2662, −844], P = 0.001). EELI values returned to baseline 10 min after closed suction and remained above baseline (mean EELI at 10, 20, and 30 min: 110, 158, and 247 impedance units above baseline, respectively). However, EELI values did not reach baseline even 30 min after open suction (mean ∆EELI at 10, 20, and 30 min: −548, −300, and −182 impedance units) (Table 2). The mean changes in lung impedance (ΔEELI) at each time point after open and closed suction are shown in Fig. 2.
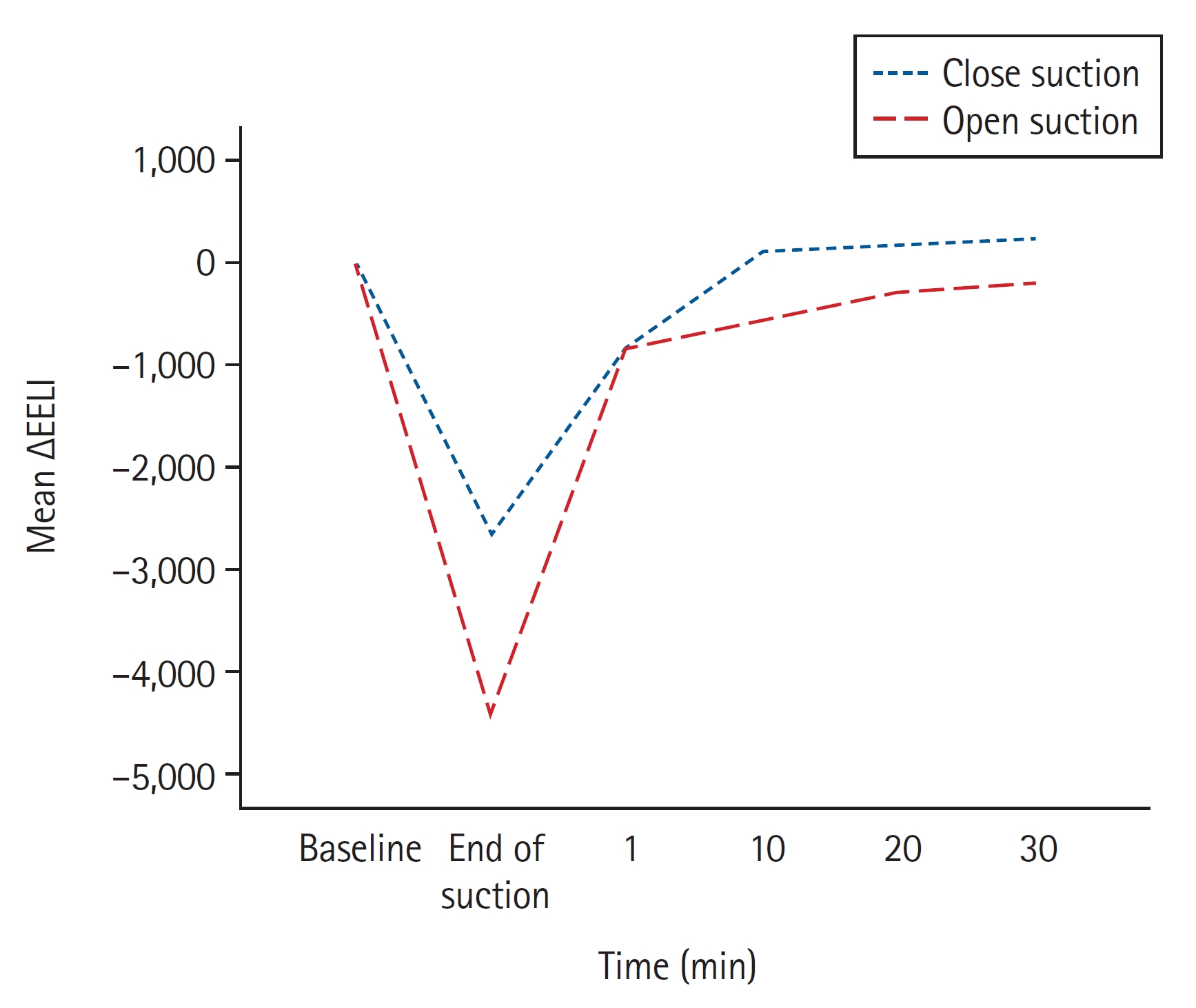
Mean changes in end-expiratory lung impedance (EELI) after open and closed suction. Open suction caused greater end-expiratory volume loss than closed suction. EELI reached baseline after 10 min of closed suction but did not reach baseline after 30 min of open suction.
The baseline mechanical ventilatory parameters were similar between open and closed suction. However, 30 min after aspiration, the Pplat and Pdrive decreased and CRS increased under closed suction, whereas after open suction, the Pplat and Pdrive increased and CRS decreased (Table 3). Arterial blood gas parameters were not significantly different 30 min after aspiration in either group, except for the PaO2/FiO2 ratio, which increased slightly after closed suction (median ΔPaO2/FiO2: 15 vs. 2; P = 0.016).
Discussion
Our study showed that open suction resulted in greater volume loss than closed suction in patients with ARDS. The EELI returned to baseline values 10 min after closed suction and remained above the baseline; however, even 30 min after open suction, baseline values were not achieved. These results indicate that closed suction is more protective against EELV than open suction in patients with ARDS.
We found that closed suction led to less volume loss at the end of expiration than open suction. In previous studies, open suction was associated with greater volume loss in postoperative patients and those with lung injury [5,7,11]. Our findings are thus consistent with those of previous studies. However, our findings also showed slower recovery of the EELV after closed aspiration. Corley et al. [7] reported that although less volume loss was found with closed suction, the EELV recovered more slowly with closed suction than with open suction. By contrast, we found that EELV recovery was faster with closed suction than with open suction. This may be explained by a couple of factors. First, the participants in our study were ARDS patients whose alveoli tended to collapse, while the participants in Corley et al. [7]’s study were post-cardiac surgery patients who may have had relatively better lung conditions at baseline. Second, the participants in our study were ventilated with higher PEEP levels than those in the study conducted by Corley et al. Opening the lungs to atmospheric pressure during open suction may have caused more volume loss owing to the release effect in ventilated patients with a higher PEEP level than those with a lower PEEP level. Third, recruitment of collapsed alveoli due to the loss of EELV may be more difficult in patients with ARDS than in postoperative patients.
We found that respiratory parameters, such as the Pplat, Pdrive, and CRS, improved 30 min after closed aspiration. We also found that open suction negatively affected these ventilatory parameters. Although closed aspiration has been associated with less EELV loss, the effect of closed aspiration on ventilator parameters could not be observed in previous studies. Cereda et al. [5] found similar ventilatory parameters before and after closed and open suction. A greater loss of EELV after open suction may lead to greater alveolar derecruitment. More alveoli collapsed owing to higher volume loss during open suction, and some may have remained collapsed after open suction. Therefore, the tidal volume may have been distributed to fewer alveoli compared to baseline, which may have resulted in the worsening of ventilator parameters with open suction.
Oxygenation did not change after open suction. The oxygen saturation and PaO2 at baseline and 30 min after open suction were similar. We observed that oxygenation improved after closed suction. The change in the PaO2/FiO2 ratio was higher with closed compared to open suction at 30 min post-aspiration. Cereda et al. [5] reported that oxygen saturation decreases rapidly after open aspiration in patients with acute lung injury but does not change during closed aspiration. However, Cereda et al. did not apply any hyperoxygenation or hyperinflation maneuvers prior to suction. A prospective crossover study conducted by Demir et al. [12] found a significant decrease in SaO2 and PaO2 after endotracheal aspiration without preoxygenation compared with endotracheal aspiration with preoxygenation. In our study, we applied hyperoxygenation before both open and closed suction; therefore, the better PaO2/FiO2 ratio observed after closed suction may have been due to the prevention of alveolar collapse and alveolar recruitment due to secretion clearance.
Our results support the use of closed aspiration in patients with ARDS, especially at higher PEEP levels. The results of previous studies on the effect of closed suction on EELV have been inconsistent. Fernandez et al. [11] showed that closed suction causes less volume loss than open suction, and that volume loss caused by closed suction recovered after 10 min. Heinze et al. [6] found that the FRC remained low 20 min after aspiration. Differences in patient populations, aspiration times, and lung volume measurement methods complicates accurate comparisons of these results. Although the differences between closed and open aspiration were relatively small, the cumulative effect may be greater because endotracheal aspiration is more frequently performed. Because the application of a closed suction system is not a costly maneuver, it is worth considering the potential benefits.
Our study had some limitations. First, this was a crossover study with a small number of participants. Because we did not use the open-circuit nitrogen washout maneuver or spirometry to measure EELV, we could not report the absolute change in EELV. Rather, we used the ΔEELI as a surrogate for the ΔEELV because a strong and linear association between the ΔEELI and ΔEELV has been shown [9]. The EELV can be computed using the EELI; however, the EELV calculated from EIT may result in an over- or underestimation of the EELV compared to that with the nitrogen washout technique [13]. The cause of ARDS in all patients was pneumonia; therefore, the effect of the aspiration method on EELV may differ in patients with ARDS due to extrapulmonary pathologies. Additionally, we focused on the effect that the suctioning method had on EELV and ventilatory parameters in the short term; however, long-term outcomes remain unclear.
This study has several implications for clinical practice. Patients with ARDS who are ventilated with higher levels of PEEP and closed suction may be less susceptible to EELV loss and worsening of other ventilatory parameters.
In conclusion, endotracheal aspiration may lead to alveolar collapse due to the loss of EELV Instead of open suction, closed suction may be a better alternative for patients with ARDS because it results in less volume loss at end-expiration without worsening of the ventilatory parameters.
Notes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Süleyman Yildirim (Conceptualization; Methodology; Project administration; Writing – original draft; Writing – review & editing)
Saba Mukaddes Saygili (Conceptualization; Data curation; Formal analysis)
Onur Süneçli (Conceptualization; Data curation; Formal analysis; Visualization)
Cenk Kirakli (Conceptualization; Methodology; Supervision; Writing – original draft; Writing – review & editing)