Does intravenous patient-controlled analgesia or continuous block prevent rebound pain following infraclavicular brachial plexus block after distal radius fracture fixation? A prospective randomized controlled trial
Article information
Abstract
Background
The purpose of this study was to investigate the role of opioid-based intravenous patient-controlled analgesia (IV PCA) or continuous brachial plexus block (BPB) in controlling rebound pain after distal radius fracture (DRF) fixation under BPB as well as total opioid consumption.
Methods
A total of 66 patients undergoing surgical treatment for a displaced DRF with volar plate fixation were randomized to receive a single infraclavicular BPB (BPB only group) (n = 22), a single infraclavicular BPB with IV PCA (IV PCA group) (n = 22), or a single infraclavicular BPB with continuous infraclavicular BPB (continuous block group) (n = 22). The visual analog scale (VAS) for pain and the amount of pain medication were recorded at 4, 6, 9, 12, 24, and 48 h and two weeks postoperatively.
Results
At postoperative 9 h, the pain VAS score was significantly higher in the BPB only group (median: 2; Q1, Q3 [1, 3]) than in the IV PCA (0 [0, 1.8], P = 0.006) and continuous block groups (0 [0, 0.5], P = 0.009). At postoperative 12 h, the pain VAS score was significantly higher in the BPB only group (3 [3, 4]) than in the continuous block group (0.5 [0, 3], P = 0.004). The total opioid equivalent consumption (OEC) was significantly higher in the IV PCA group (350.3 [282.1, 461.3]) than in the BPB only group (37.5 [22.5, 75], P < 0.001) and continuous block group (30 [15, 75], P < 0.001); however, OEC was not significantly different between the BPB only group and the continuous block group (P = 0.595).
Conclusions
Although continuous infraclavicular BPB did not reduce total opioid consumption compared to BPB only, this method is effective for controlling rebound pain at postoperative 9 and 12 h following DRF fixation under BPB.
Introduction
Distal radius fractures (DRFs) account for up to 15% of all extremity fractures [1]. Open reduction and internal fixation using volar locking anatomical plate and screws is the most frequently performed surgical procedure [2,3]. Poor pain control in the acute postoperative period is associated with patient dissatisfaction after operation [4].
Operative treatments of extremity fractures under regional anesthesia have several advantages as compared with general anesthesia, including muscle relaxation and analgesia in the acute postoperative period without the requirement of tracheal intubation that is relevant to patients with underlying lung diseases. Some studies reported that it could prevent postoperative nausea and vomiting and shorten the post-anesthesia care unit (PACU) stay duration [4–6]. In the operative treatment of DRFs, regional anesthesia is better than general anesthesia in reducing postoperative pain and pain medication use [7,8].
Despite the advantages of regional anesthesia, patient dissatisfaction may be attributed to severe pain after the regional anesthesia wears off that is known as “rebound pain”[9]. Rebound pain typically occurs in the 8 to 24 h postoperative period and is often treated with preemptive oral pain medication [10,11]. However, as the timing and intensity of rebound pain are different among individuals, oral medication may not control the pain properly or may cause opioid-related complications due to overuse [4]. Intravenous patient-controlled analgesia (IV PCA) and continuous regional block with an infusion pump would be alternative methods for controlling rebound pain; however, limited studies have been conducted on the control of rebound pain after regional block for DRF fixation.
The purpose of the current randomized controlled trial (RCT) was to investigate the role of IV PCA or continuous brachial plexus block (BPB) in controlling rebound pain after DRF fixation under BPB as well as total opioid consumption.
Materials and Methods
Patients
We performed a randomized controlled study at a single center between December 2018 and April 2019. The study was approved by our Institutional Review Board (Approval number: AMC-2018-1335), registered at the Clinical Research Institution Service (CRIS; Registration number: KCT0003404) and adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines. This study was also conducted in accordance with the ethical principles of the Helsinki Declaration 2013. All patients who underwent treatment for a displaced DRF with volar plate fixation (fracture type A, B, or C according to the AO Foundation/Orthopaedic Trauma Association classification system, as examined on radiographs) were assessed for eligibility. The inclusion criteria were age of 18–79 years and the occurrence of DRF within two weeks prior to surgery. The exclusion criteria included a concomitant ulnar fracture proximal to the base of the styloid process; a complex distal radial fracture requiring additional fixation or bone graft; previous ipsilateral wrist or hand dysfunction; previous pain disorder; concomitant nerve, tendon, or skin injury in the fractured wrist; concomitant injury at other sites requiring additional surgery and/or pain medication; ongoing drug or alcohol abuse; severe psychiatric disorder; or systemic inflammatory diseases. Patients who met all the inclusion and exclusion criteria were informed about the study and offered to participate in the study by an orthopedic surgeon. After written consent was obtained, the patients were randomized to receive a single infraclavicular BPB (BPB only group), a single infraclavicular BPB with opioid-based IV PCA (IV PCA group), or a single infraclavicular BPB with continuous infraclavicular BPB (continuous block group) through block randomization (n = 6) using sequentially numbered closed opaque envelopes (Fig. 1).
Interventions
All anesthetic and surgical interventions were performed with standardized protocols. All peripheral nerve block procedures were performed under ultrasonography guidance (Logiq P9; GE Healthcare, USA) by two anesthesiologists with more than five years of experience with peripheral nerve block. After the confirmation of the posterior cord with electrical stimulation, 0.4 to 0.6 ml/kg of the prepared 0.375% ropivacaine, consisting of a mixture of 20 ml 0.75% ropivacaine (Kabiropivacaine; Fresenius Kabi, Norway) and 20 ml normal saline, was administered to all the patients. In the continuous block group, echogenic catheter-over-needle (E-Cath PLUS; PAJUNK, Germany) was used. After the single-block procedure, the needle was removed, and the tip of the catheter remained between the axillary artery and the posterior cord. The catheter was secured to the skin with adhesive tapes when it was properly positioned. The sensory and motor blockades of the patients were evaluated 30 min after the BPB procedure. After confirmation of a successful block, a dose of 1 μg/kg intravenous dexmedetomidine was loaded over 10–15 min, followed by a continuous infusion of 0.5–1.0 μg/kg/h until the end of the operation.
For the IV PCA group, the analgesic was prepared with a mixture of fentanyl citrate (Hana Pharmacy, Korea) and normal saline. The total volume was set to 100 ml; however, the fentanyl citrate dose was determined based on each patient’s body weight as follows: < 50 kg, 1000 μg; 50–70 kg, 1200 μg; > 70 kg, 1500 μg. The PCA pump (AutoMed 3200, Ace Medical, Korea) was set for a basal rate of 1 ml/h, a bolus dose of 1 ml, and a locking time of 15 min. For the continuous block group, 250 ml 0.15% ropivacaine, consisting of a mixture of 50 ml 0.75% ropivacaine and 200 ml normal saline, was administered as follows: a basal rate of 5 ml/h, a bolus dose of 5 ml, and lockout time of 30 min. The infusion pump was started just before the end of the operation in both groups.
The patients underwent surgery with a volar Henry approach to the distal part of the radius, followed by open reduction and internal fixation with a single volar locking plate (Synthes, Switzerland). All operations were performed by a fellowship-trained orthopedic surgeon. Until two weeks postoperatively, a volar short arm splint was applied.
Postoperatively, the patients were monitored in the PACU for 1–2 h and transferred to the general ward. The patients were instructed to call a nurse for pain medication (1–2 pills of 5 mg of oxycodone hydrochloride [HCL]) every 4–6 h as needed. The pain level was measured by an on-duty nurse with a visual analog scale (VAS; 0 to 10) at 4, 6, 9, 12, 24, and 48 h after surgery. When the timing of pain measurement and medication requirement were similar, pain was measured just before taking medication. IV PCA or infusion pump for continuous BPB was discontinued at 48 h after surgery. If the pump was empty before 48 h, it was discontinued before 48 h. In addition, when hand motor paralysis persisted over 24 h in the continuous block group, the catheter was removed before 48 h to prevent hand stiffness. The patients were discharged on the third day after the operation with 30 pills of 5 mg oxycodone HCL and were instructed to take 1–2 pills every 4–6 h as needed. They were followed up at two weeks after operation for stitches out with an assessment of the pain level and the amount of medication taken.
Methods of assessment
The primary outcome was pain as measured with a VAS at 12 h after operation. The secondary outcome was the total opioid equivalent consumption (OEC) during the two weeks after operation. The VAS score for pain and the amount of pain medication were recorded at 4, 6, 9, 12, 24, and 48 h and two weeks after the operation. The total amount of infused IV PCA or continuous BPB for 48 h after operation and total opioid consumption for two weeks after operation were assessed. All opioid analgesics were converted to opioid equivalents (milligrams of oral morphine). Any postoperative analgesia-related complications were evaluated.
Statistical analysis
To determine the statistical power, the VAS score for pain at 12 h after surgery was used as the primary outcome variable. In a pilot study of 15 patients (five patients in each group), the mean VAS score for pain was 5.2 ± 2.3 in the BPB only group, 3.2 ± 1.1 in the BPB with IV PCA group, and 4.0 ± 2.2 in the continuous block group at 12 h after operation. On the basis of these results, a power analysis revealed that a sample size of 20 patients per group would provide 80% statistical power to detect this effect size between the groups (alpha = 0.05, beta = 0.20) with analysis of variance. To account for a possible follow-up loss of 10%, we aimed to enroll 22 patients in each group (a total sample size of 66 patients).
The characteristics of the patients, including age and body mass index, VAS score for pain and oral medication at each time point, and total opioid consumption, were determined using the Kruskal-Wallis test. The Mann-Whitney U test was used for the post hoc analysis of between-group comparisons to allow for the number of comparisons performed (three comparisons for each variable). The sex ratio, American Society of Anesthesiologists classification, and fracture type distribution of the patients were compared using the Fisher’s exact test.
Results
Patient enrollment
A total of 181 wrists (180 patients) were assessed for eligibility during the study period; 66 wrists (66 patients) were included in the study and randomized to the BPB only (22 wrists), IV PCA (22 wrists), or continuous block group (22 wrists). The baseline characteristics are presented in Table 1. Among the three groups, the patients in the continuous block group were significantly younger; however, this was not statistically significant in the post hoc analysis of the between-group comparisons.

Baseline Information of the Patients Randomized to the BPB Only, IV PCA, and Continuous Block Groups
Two patients in the continuous block group were excluded because the infusion pump did not function properly due to catheter migration within 12 h after operation. One patient in the BPB only group required additional intravenous opioid analgesia after oral medication and two patients in the BPB only group and another two patients in the continuous block group required additional oral oxycodone HCL administration after taking 10 mg of oral oxycodone HCL between 9 and 12 h after operation. These five patients were included in the analysis. Finally, 64 patients completed the follow-up until two weeks postoperatively.
Postoperative pain
At 9 h after operation, the VAS score for pain was significantly higher in the BPB only group (median: 2; Q1, Q3 [1, 3]) than in the IV PCA (0 [0, 1.8], P = 0.006) and continuous block groups (0 [0, 0.5], P = 0.009). At 12 h after operation, the VAS score for pain was significantly higher in the BPB-only group (3 [3, 4]) than in the continuous block group (0.5 [0, 3], P = 0.004). The median pain scores at other time points did not differ significantly among the three groups (Fig. 2 and Supplementary Table 1).
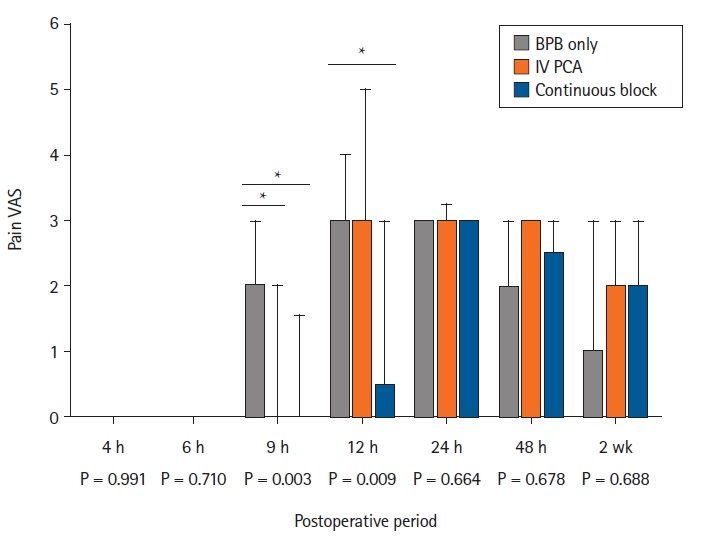
Box plot showing the median VAS score for pain in the two-week postoperative period for 66 patients randomized to the following groups: BPB (BPB only group), single infraclavicular BPB with IV PCA (IV PCA group), and single infraclavicular BPB with continuous infraclavicular BPB (continuous block group). BPB: brachial plexus block, IV PCA: intravenous patient-controlled analgesia, VAS: visual analog scale. The horizontal bar indicates the median, and the upper bound indicates the third quartile. *P < 0.017.
Postoperative OEC
From 9 to 12 h after operation, the OEC was significantly higher in the BPB only group (7.5 [7.5, 13.1]) than in the IV PCA (0 [0, 5.6], P = 0.001) and continuous block groups (0 [0, 1.9], P = 0.013) (Fig. 3). From 48 h to two weeks after operation, the OEC was significantly higher in the IV PCA group (48.8 [16.9, 103.1]) than in the BPB only (0 [0, 28.1], P = 0.003) and continuous block groups (11.3 [0, 39.4], P = 0.010). The total OEC including IV PCA during two weeks after operation was significantly higher in the IV PCA group (350.3 [282.1, 461.3]) than in the BPB only (37.5 [22.5, 75], P < 0.001) and continuous block groups (30 [15, 75], P < 0.001). The total OEC was not significantly different between the BPB only and the continuous block groups (P = 0.595, Supplementary Table 2).
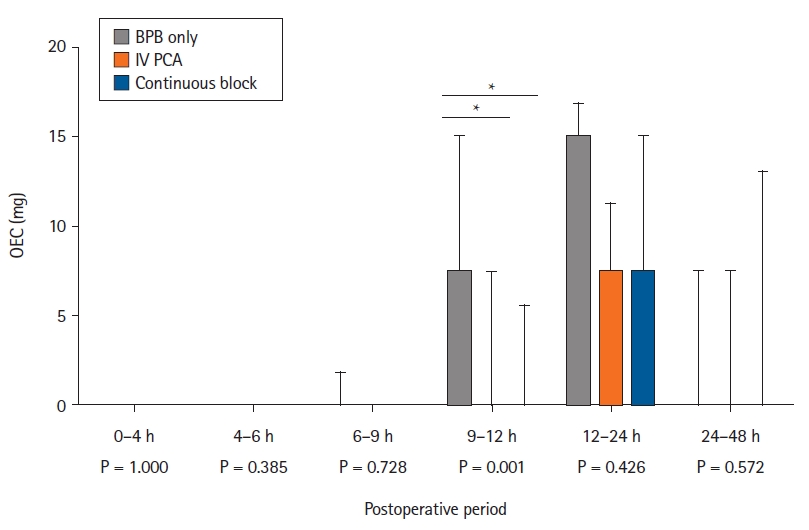
Box plot showing the oral OEC in the 48 h postoperative period for 66 patients randomized to the following groups: BPB (BPB only group), single infraclavicular BPB with intravenous patient-controlled analgesia IV PCA (IV PCA group), and single infraclavicular BPB with continuous infraclavicular BPB (continuous block group). BPB: brachial plexus block, IV PCA: intravenous patient-controlled analgesia, OEC: opioid equivalent consumption. The horizontal bar indicates the median, and the upper bound indicates the third quartile. *P < 0.017.
Complications
Two patients in the IV PCA group (9.1% of 22 patients) experienced nausea and vomiting from 12 to 24 h after the operation and IV PCA was stopped for a few hours after taking an anti-emetic medication. One patient (5.0% of 20 patients) in the continuous block group had constipation, and a laxative was prescribed. Seven patients in the continuous block group (35.0% of 20 patients) had motor paralysis persisting over 24 h after operation, and the catheter was removed before 48 h after operation. They were encouraged to perform active-assisted range-of-motion exercise for all fingers and did not have hand stiffness at two weeks after operation.
Discussion
Rebound pain could be controlled by timed pain medication in the wear-off period of regional anesthesia; however, correctly timed pain medication at an appropriate level is difficult for several reasons. First, the timing of rebound pain varies even with the same type of operation, for example, 12–24 h for extremity fracture fixation [9,12], and 1–2 days for shoulder arthroscopy [13]. In addition, depending on the operation time and patient condition, the duration of regional block would be changed [12]. Second, patients are often reluctant to take pain medications especially opioids when they are not yet in pain [12]. Third, the extent of rebound pain varies among patients who underwent the same procedure. After DRF fixation under BPB, one study reported a median VAS score for pain of 3 with an IQR of 3, but another study described a mean VAS score for pain of 5.5 with a SD of 2.4 at the same time point (24 h after operation) [7,8]. Therefore, the amount of pain medication required is not predictable, and inadequate prediction could lead to the abuse or overdose of pain medication without the proper pain management. This RCT revealed that instead of oral pain medication, continuous infraclavicular BPB reduced the intensity and duration of rebound pain in the wear-off period of BPB and had a total OEC similar to that in BPB only.
The role of continuous block for the control of rebound pain after regional block was demonstrated in various extremity surgeries. Continuous interscalene block showed better pain control and a lesser requirement of pain medication than single block in shoulder surgery in several RCTs [14–16]. After anterior cruciate ligament reconstruction, continuous femoral nerve block showed longer pain-free time and lesser rebound pain than single block [17]. In an RCT for patients with ankle fracture fixation, continuous popliteal sciatic nerve block showed lesser rebound pain and opioid consumption than single block [12]. However, in an RCT for patients with DRF fixation, postoperative pain was not significantly different between the continuous infraclavicular block and the single block [9]. Several limitations existed in the previous RCT to consider it as a final report about the effect of continuous block in patients with DRF fixation. Randomization between the continuous and single blocks was partial due to logistical barriers, pain medication in the PACU was not recorded, and pain scores were recorded by the patients themselves that means the pain score at the current time point could be influenced by the numerical value at previous time points.
Catheter migration could occur both in the lower and upper extremities and is an obstacle for the clinical use of continuous block. After continuous popliteal sciatic nerve block for pain control after ankle fracture surgery, 5 of 23 patients (21.7%) experienced an unintentional dislodgement of their catheter during the early postoperative period [12]. After continuous interscalene block for pain control after rotator cuff repair surgery, 1 of 22 patients (4.5%) experienced an accidental removal of their catheter [18]. In our study, 2 of 22 patients (9.1%) revealed catheter migration at an unknown timing. The cause of catheter migration is unclear [19], but temporary motor paralysis of the involved extremities that could not be patient-controlled could increase the risk of unintentional catheter migration. Therefore, clinicians should explain the possibility of catheter migration to the patients and advise against vigorous movement of their extremities during the acute postoperative period.
Delayed sensory and motor recovery after continuous block is an inevitable complication. Several studies informed the risk of fall in patients with continuous femoral nerve block and pressure injury of insensate extremities [19]. Concentration, volume, and infusion rate of continuous block could influence the preservation of more motor function and proprioception, but the correct relationship is unclear and different depending on the anatomic locations [20]. We utilized continuous infraclavicular BPB with relatively low concentration—0.15% ropivacaine—to minimize the risk of prolonged insensation and paralysis. However, a considerable number of patients had motor paralysis persisting over 24 h after operation. Patients could experience anxiety about their paralyzed extremity, but explanation in advance and reassurance could relieve this anxiety. In addition, prompt catheter removal reversed the paralysis, and acute postoperative pain was not an issue after 24 h. Hand and finger stiffness could occur after paralysis, but it could be controlled by active-assisted range-of-motion exercise for all fingers and no sequelae remained in our patients.
Prescription opioid abuse is an increasing problem and has been associated with an increase in opioid overdose-related deaths [21,22]. Orthopedic surgeons represent the third largest group of opioid prescribers in the United States [23], and upper extremity surgeons tend to overprescribe opioids for postoperative pain control [24]. Therefore, development of a protocol to control pain after DRF fixation, which is a common procedure, with minimal OEC is important. In this study, the IV PCA group showed significantly lower pain VAS scores at postoperative 9 h with significantly lower OEC between postoperative 9 and 12 h than the BPB only group. In addition, the complication rate was lower than that of the continuous block group. However, compared with other groups, more oral opioids were required after the acute postoperative period between 48 h and two weeks. This phenomenon may be attributed to prior continuous opioid infusion during the acute postoperative period (opioid tolerance) [25].
This study has several limitations. First, all operations were performed after hospitalization that may not reflect the reality of many institutes performing DRF fixation ambulatory surgery. However, we think that the outcome variables, including the pain level at each time point, timing and amount of oral medication, and infused dosage of IV PCA or continuous block, could be assessed more accurately in the hospitalization setting. Second, postoperative pain levels determined on the basis of VAS scores are subjective and might be influenced by psychological factors and personal experience. In addition, pain level was evaluated by different on-duty nurses and not by a single evaluator in this study. Third, intra-venous dexmedetomidine that was used in this study for patient sedation could influence the acute postoperative pain and the amount of required pain medication. Fourth, all the study participants, including the orthopedic surgeon, anesthesiologists, duty nurses, and patients, were not blinded to the type of additional pain control after BPB. Finally, we did not use any adjuvants such as dexamethasone. Several studies revealed that the use of dexamethasone could prolong the duration of the nerve block and reduce rebound pain [26–28]. A well-designed further study is required to compare the effects of catheterization and dexamethasone on rebound pain and cost-effectiveness.
In conclusion, our data suggest that continuous infraclavicular BPB reduced the intensity and duration of rebound pain in the wear-off period of BPB. In addition, the total OEC was similar to that in the BPB only group. Although continuous infraclavicular BPB did not reduce total opioid consumption compared to BPB only, this method is effective for controlling rebound pain at postoperative 9 and 12 h following DRF fixation under BPB.
Notes
Funding
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, and the Ministry of Food and Drug Safety) (KMDF_PR_20200901_0039).
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Author Contributions
Jong-hyuk Lee (Conceptualization; Investigation; Methodology; Project administration; Resources; Writing – review & editing)
Ha-jung Kim (Conceptualization; Investigation; Methodology; Project administration; Resources; Writing – review & editing)
Jae Kwang Kim (Methodology; Project administration; Resources; Writing – review & editing)
Sungjoo Cheon (Investigation; Methodology; Resources; Validation)
Young Ho Shin (Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Visualization; Writing – original draft; Writing – review & editing)
Supplementary Materials
Visual analogue scale scores for postoperative pain.
Median opioid equivalent consumption (OEC) in the 2-week postoperative period.

