Inadvertent free flow of remifentanil caused by a damaged syringe installed in syringe pump
Article information
Infusion pumps are extensively used in clinical settings to administer fluids and drugs. Syringe pumps are especially useful because they are easy to prepare and convenient when small volumes of liquid medications need to be infused over a relatively long duration [1]. Despite the use of syringe pumps in everyday practice, the safe use of infusion devices is often overlooked by medical staff. Numerous hazards of infusion pumps have been reported [1–3].
The requirement for patient consent was waived by the Institutional Review Board of our institution (H-2003-019-089). A 32-year-old healthy pregnant woman was scheduled for emergency cesarean section. She was at 37+5 weeks of gestation and had undergone cesarean section four years ago. The preoperative examinations revealed normal results. After providing informed consent for anesthesia, the patient entered the operation room, and routine monitoring was performed. Her vital signs were stable. An anesthesia nurse installed a 20-ml syringe containing remifentanil (0.1 mg/ml) on a syringe pump (Injectoma TIVA Agilia®, Fresenius Kabi, Germany) and connected it to an intravenous line via a three-way stopcock. The syringe pump was powered off because the patient needed to be sterilized and draped before the induction of anesthesia.
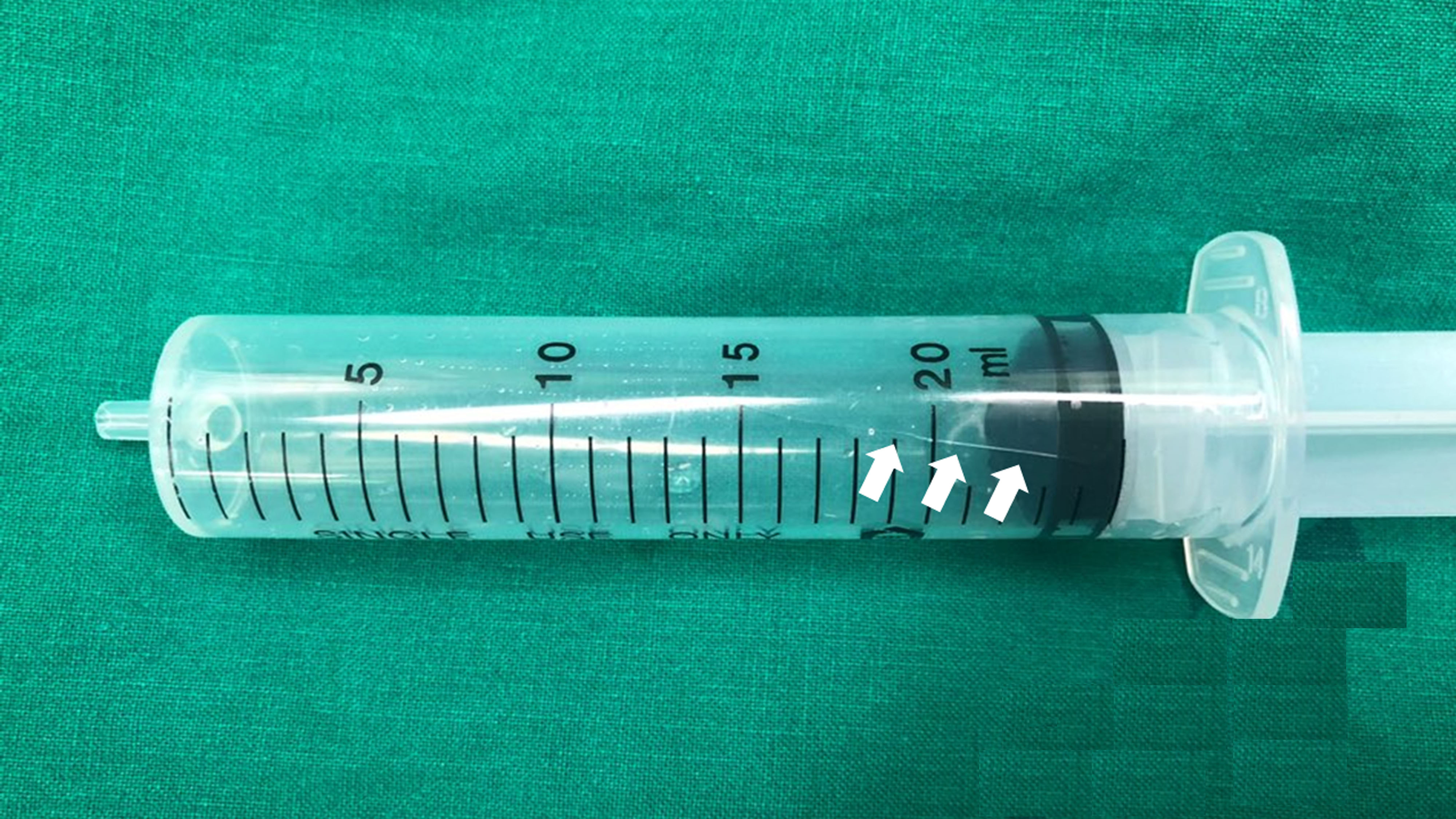
After a few minutes, the patient closed her eyes, and an anesthesiologist asked her to open her eyes to confirm consciousness. She opened her eyes immediately but closed them soon again. Then, upward ocular deviation occurred. She did not respond to any command and became apneic. Flat capnography was observed and the Bispectral index fell to 75 at the same time. Then, SpO2 dropped to 89%, and mask bagging with 100% O2 was started. At that time, her blood pressure and heart rate were 99/56 mmHg and 75 beats/min, respectively, and SpO2 was quickly recovered to 100% after applying mask ventilation. We attempted to determine the reason for her unconsciousness and checked the syringe pump. A crack in the syringe was noted, through which air bubbles had entered and pushed the remifentanil solution into the patient (Fig. 1, Video 1). We immediately disconnected the remifentanil solution from the patient’s intravenous line. Consequently, only 10 ml of remifentanil solution remained in the syringe. After a brief notice to the obstetrician, 250 mg of thiopental sodium and 40 mg of rocuronium were injected. Tracheal intubation was performed without difficulty, and 5 min later, a male baby was delivered. No other notable events occurred during the remaining intraoperative period. The patient and her baby were discharged a week later.
After the events, the distribution and storage of the syringe were investigated, but there were no problems. We tested 10 more syringes installed in the same syringe pump, and all of them were similarly damaged. Also, the syringes were not damaged in other syringe pumps of the same model. The syringe pump was sent to the department of bioengineering in our hospital. They found that the syringe pump was aged and the clamp did not lock gently but became instantaneously pressurized and broke the syringe during installation. We did not report this case to the Korean patient safety reporting and learning system (KOPS, https://www.kops.or.kr) because we thought the problem was isolated to this particular syringe pump. However, two months later, the same adverse event occurred with another syringe pump of the same model in the surgical intensive care unit. Free flow of 1.5 mg of remifentanil was noticed after hypotension and bradycardia became apparent in a 93-year-old female patient. Her vital signs immediately returned to normal after dopamine infusion. We have reported this case to KOPS because we supposed that other similar cases may occur in the future.
This case raises several important points. Firstly, there is no consensus regarding how long syringe pumps should be used in terms of safety and cost-effectiveness. The two pumps have been used for seven years without any problems before this event. Old medical devices have the potential to malfunction at any time without any warning signs. Often, aging medical devices are discarded only after an adverse event occurs in the patient that ranges from minor to fatal. Another important point is patient monitoring during the use of infusion pump [3]. Although the syringe pump was placed only approximately 5 cm higher than the patient, air entered through a crack by siphon effect. The three-way stopcock connecting to the syringe pump was opened, and it did not have an anti-siphoning valve in this case. Both allowed free flow of remifentanil to the patient. The alarm did not sound because the syringe pump was not powered on. Moreover, the damaged syringe was difficult to be noticed because the crack was small and partially covered by the clamp. Therefore, if the patient was left without monitoring, unconsciousness and apnea could have gone unnoticed that would have led to a fatal consequence in both the mother and the baby.
It is necessary to actively report medical device adverse events. In the first case, we did not report it because we thought it was only a problem with the syringe pump used in this case. Two months later, the same problem occurred, and the second case had almost gone unreported as well; however, the anesthesiologist who was involved in the second case had heard about the first case. The clinical trials for medical devices are conducted under strictly controlled conditions, so it is difficult to say that the safety of medical devices is completely evaluated in these trials. Therefore, to supplement the limitations of pre-market evaluations of medical device safety, the post-market surveillance system continues to manage medical device adverse events even after the device is licensed [3,4]. However, there are various obstacles for medical staff or manufacturers to voluntarily report medical device adverse events to the reporting system, including fear of blame, lack of time, perceived ineffectiveness of reporting, complexity of reporting, and lack of knowledge of the reporting system [5]. Therefore, it is necessary to encourage the idea that voluntary reporting is important for patient safety and the improvement and development of medical device performance and to provide education on how to report adverse events to the reporting system.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding Statement
This work was supported by clinical research grant in 2020 from Pusan National University Hospital.
Author Contributions
Hyea-Jin Kim (Funding acquisition; Writing – original draft; Writing – review & editing)
Ah-Reum Cho (Conceptualization; Writing – review & editing)
Daehoan Moon (Data curation; Writing – review & editing)
Ji-Youn Lee (Data curation; Writing – review & editing)
Hyewon Hwang (Writing – review & editing)
Supplementary Material
Video 1.
After the event, remifentanil was discarded and 20 ml of normal saline solution was filled into the damaged syringe and connected to an intravenous line of the patient. Air bubbles entering the syringe and pushing the solution into the patient, even when the power is off.