Driving pressure guided ventilation
Article information
Abstract
Protective ventilation is a prevailing ventilatory strategy these days and is comprised of small tidal volume, limited inspiratory pressure, and application of positive end-expiratory pressure (PEEP). However, several retrospective studies recently suggested that tidal volume, inspiratory pressure, and PEEP are not related to patient outcomes, or only related when they influence the driving pressure. Therefore, this review introduces the concept of driving pressure and looks into the possibility of driving pressure-guided ventilation as a new ventilatory strategy, especially in thoracic surgery where postoperative pulmonary complications are common, and thus, lung protection is of utmost importance.
Introduction
Postoperative pulmonary complications are not rare in thoracic surgery due to direct surgical injury of the lung tissues and one lung ventilation which is prone to volutrauma, barotrauma, atelectrauma, and oxygen toxicity [1-3]. In addition, immune cells are abundant on the pulmonary vascular endothelium and alveolus [4], thus, direct and indirect injuries to the lung tissues trigger a profound inflammatory response and increase pulmonary vascular permeability in both dependent and non-dependent lungs [5]. These reactions often precede systemic inflammatory response syndrome, acute respiratory distress syndrome (ARDS), and pneumonia [6-8]. Therefore, lung protection is of utmost importance, and protective ventilation is strongly recommended in thoracic surgery [9,10].
The usual settings for protective ventilation during one lung ventilation are tidal volume (VT) 5 to 6 ml/kg of predicted body weight (PBW), positive end-expiratory pressure (PEEP) to 5 cmH2O and plateau pressure (Pplat) to less than 25 cmH2O [9–13]. However, a high incidence of postoperative pulmonary complications is still being observed even with a protective ventilatory strategy [3,12,14-16].
Driving pressure was first introduced by Amato et al [17] in 2015 in their meta-analysis study for ARDS patients. The authors suggested that high driving pressure was most strongly associated with worse survival. VT, Pplat and PEEP were not related to patient outcomes, or only related when they influenced the driving pressure. Several retrospective and prospective studies confirmed the importance of driving pressure in ARDS patients [18,19] and surgical patients [12,20-22].
This review article introduces the concept of driving pressure through previous publications, and will discuss the possibility of driving pressure-guided ventilation as a new ventilatory strategy in surgical patients including thoracic surgery.
Driving pressure
Definition
Driving pressure is [Pplat – PEEP] and is the pressure required for the alveolar opening [17]. Static lung compliance (Cstat) is expressed as [VT / (Pplat – PEEP)]. Thus, driving pressure is also expressed as [VT / Cstat]. Driving pressure has an inverse relationship with Cstat and an orthodromic relationship with VT according to this formula. High driving pressure indicates poor lung condition with decreased lung compliance.
Previous studies on driving pressure
Retrospective studies for ARDS patients
Most studies regarding driving pressure were retrospective studies for ARDS patients. Following Amato’s meta-analysis [17], subsequent retrospective studies also showed that driving pressure is more strongly associated with survival than VT and PEEP in ARDS patients [18]. Driving pressure was closely related to hospital mortality even among patients who received protective ventilation [19]. The cut-off value of driving pressure for high mortality was approximately 15 cmH2O for ARDS patients [17,23], and each unit increase of driving pressure (1 cmH2O) was associated with a 5% increment in mortality [18].
High driving pressure was also associated with increased mortality in patients receiving pressure support mode ventilation in a recent retrospective cohort study [24]. The driving pressure was higher in non-survivors than in survivors, but the difference was only 1 cmH2O [11 (9 to 14) vs. 10 (8 to 11) cmH2O; P = 0.004]. Cstat was lower [40 (30 to 50) vs. 51 (42 to 61) ml/cmH2O] in the non-survivors, but peak pressure was not different between non-survivors and survivors [all values median (IQR)]. Lower Cstat [odds ratio, 0.92 (95% CI, 0.88 to 0.96)] and higher driving pressure [odds ratio, 1.34 (95% CI, 1.12 to 1.61)] were independently associated with increased risk of death, but peak inspiratory pressure was not associated with mortality [24].
Retrospective studies for general surgical patients
For surgical patients, a meta-analysis was published based on 17 randomized controlled trials of protective ventilation (n = 2,250) [22]. In its multivariable analyses, driving pressure was associated with the development of postoperative pulmonary complications, whereas no association was found with VT and PEEP [22]. The odds ratio for postoperative pulmonary complications was 1.16 for each 1 cmH2O increase in driving pressure. In a mediator analysis, driving pressure was the only significant mediator of the effects of protective ventilation on development of pulmonary complications [22]. In its sub-group analysis, high PEEP was related to a greater risk of postoperative pulmonary complications if high PEEP increased driving pressure (OR 3.11, 95% CI 1.39 to 6.96; P = 0.006, compared to the low PEEP group). The same high PEEP trended toward decreasing the risk of pulmonary complications if it decreased the driving pressure (OR 0.19, 95% CI 0.02 to 1.50; P = 0.154, compared to the low PEEP group) [22].
In a cohort study of cardiac surgery patients published in 2019 (n = 4,694), a lung-protective ventilation bundle was applied to 1,913 patients (40.8%) [20]. Postoperative pulmonary complications were reduced by its application (13.9% vs. 6.6%, OR 0.56, 95% CI 0.42 to 0.75). This protective ventilation bundle was comprised of VT < 8 ml/kg ideal body weight, modified driving pressure [peak inspiratory pressure - PEEP] < 16 cmH2O, and PEEP ≥ 5 cmH2O. Of these components, modified driving pressure < 16 cmH2O was independently associated with decreased pulmonary complications (OR 0.51, 95% CI, 0.39 to 0.66), but VT < 8 ml/kg and PEEP ≥ 5 cmH2O were not [20].
These retrospective studies clearly show that high driving pressure is the best indicator of poor prognosis, but do not confirm that active control of driving pressure reduces complications or improves outcomes. The previous cohort study conducted in cardiac surgery patients did not give a detailed account of the technique to reduce driving pressure in their protective ventilation bundle [20].
Suggested mechanism of how driving pressure guided-ventilation can decrease morbidity
‘Functional lung size’ is the volume of aerated lung available for ventilation (Fig. 1) [25]. ‘Functional lung size’ is derived from the ‘baby lung’ concept. Computed tomography (CT) examinations showed that the ARDS patients only have the same amount of normally aerated lung tissue as a 5-6 year-old child [25]. The respiratory system compliance is linearly related to the ‘baby lung’ dimensions. Thus, the ARDS lung is not “stiff” but instead small, with nearly normal intrinsic elasticity. The ‘baby lung’ concept conveys that the VT/‘baby lung’ ratio is more important than the VT/kg ratio, and the smaller the ‘baby lung’, the greater the potential is for ventilation induced lung injury. The ‘baby lung’ is not a distinct anatomical structure. The CT density redistribution in prone position showed that the 'baby lung' is a functional but not an anatomical concept [25].

Functional lung size. ‘Functional lung size’ is different with anatomical lung size. Only aerated alveoli during ventilation (black arrow: hypoechoic alveoli) contributes to functional lung size. Areas of inflammation, collapse, fibrosis, or consolidation are not aerated properly during ventilation and do not contribute to functional lung size (white arrow: hyperechoic alveoli).
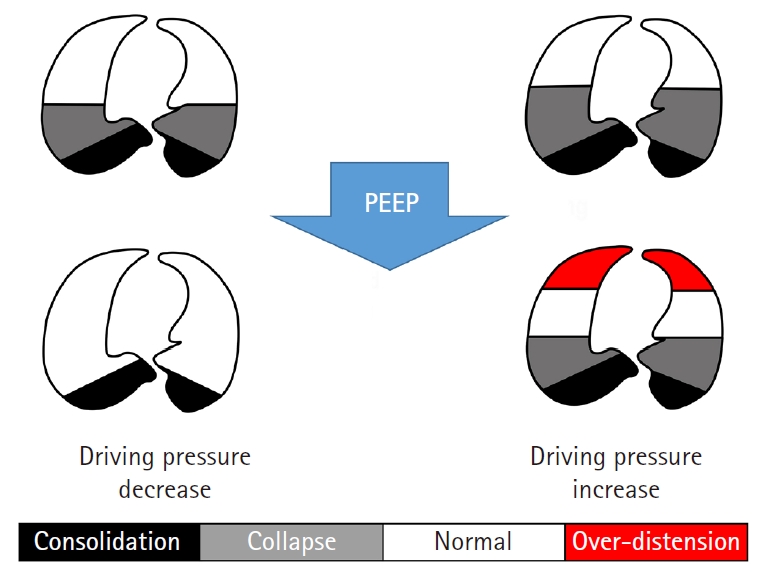
Similarly with ARDS, ‘functional lung size’ would be smaller than expected if the patients have lung pathologies such as atelectasis, consolidation, bullae, effusion, or fibrosis. Either over-distending (barotrauma) or under-ventilating (atelectrauma) the lungs beyond the functional lung size would increase driving pressure [17,25]. Driving pressure would be lowest when the PEEP maintains alveoli at the functional residual capacity at the end of expiration and VT expands the lungs within the ‘functional lung size’ [1,21,26-31]. Fig. 2 shows that ventilation occurring in the high compliance zone shows the lowest driving pressure.
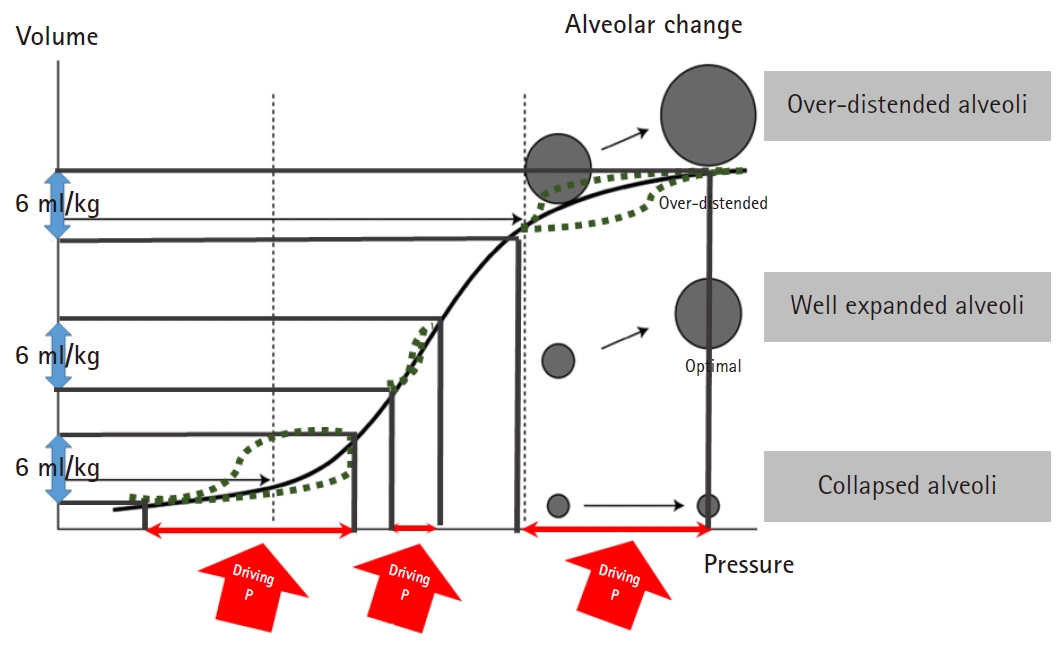
Pressure volume curve. Ventilation occurring in the high compliance zone shows the lowest driving pressure.
Therefore, driving pressure can be used to guide individualized ventilation based on each patient’s functional lung size. Technically, it is easier to use driving pressure as guidance than to use Cstat because driving pressure is the calculation of two simple pressures [Pplat – PEEP] and Cstat is the interaction of pressures and tidal volumes [VT / (Pplat – PEEP)] which is more difficult to calculate and may show more erratic changes during surgical manipulation than driving pressure.
The two methods to reduce driving pressure
There are no established techniques to reduce driving pressure yet. Driving pressure is dependent on PEEP and VT [driving pressure = Pplat – PEEP = VT / Cstat]. Therefore, adjustment of PEEP and VT has the potential to reduce driving pressure. There are few studies regarding PEEP adjustment based on driving pressure [21,30,32] and no studies yet on the VT titration based on driving pressure.
PEEP titration
Previously, high PEEP (13 to 15 cmH2O) was compared to low PEEP (8 cmH2O) in a large scale acute respiratory distress syndrome net (ARDSnet) trial and found no difference in mortality and unassisted breathing (n = 549) [33]. In a subsequent multicenter randomized controlled trial of 767 adults with acute lung injury, patients were randomly assigned to a moderate PEEP strategy (5-9 cmH2O, mean PEEP 7 cmH2O) or to a level of PEEP set to reach a Pplat of 28 to 30 cmH2O (mean PEEP 15 cmH2O). The primary outcome, 28-day mortality rate and the hospital mortality rate were not different between the two groups [34].
According to electrical impedance measurement, each patient and each lung region have different lung compliance [35]. In addition, the majority of patients with chronic obstructive pulmonary disease develop intrinsically variable PEEPs during mechanical ventilation [36]. Therefore, fixed PEEP would be inappropriate regardless of whether it is high or low, and individualized PEEP based on driving pressure may be the next step of protective ventilation. Fig. 3 shows that the same PEEP decreases or increases driving pressure according to the underlying lung pathologies or functional lung size [22,26].
For PEEP titration, most previous studies were small scale studies and PEEP was titrated using Cstat. However, PEEP titration using Cstat also decreased driving pressure. In one study of abdominal surgery patients, PEEP was titrated to yield the highest Cstat (n = 36) in the experimental, or ‘individualized PEEP’ group while the control group received fixed PEEP of 5 cmH2O [30]. Individualized PEEP decreased driving pressure by 28% compared to fixed PEEP. Mean driving pressure was 5.6 ± 1 cmH2O and 7.4 ± 1 cmH2O for the individualized PEEP group and fixed PEEP group, respectively (P < 0.001). The average PEEP was 8 cmH2O with a range from 6 to 14 cmH2O in the individualized PEEP group [30].
Pereira et al. [32] used electrical impedance tomography to determine individual PEEP such that both lung collapse and hyperdistension are minimized simultaneously in abdominal surgery (n = 40). This study showed PEEP titration reduces driving pressure compared to fixed PEEP 4 cmH2O (8.0 ± 1.7 vs. 11.6 ± 3.8 cmH2O; P < 0.001). The median PEEP was 12 cmH2O but the range varied from 6 to 16 cmH2O in the individualized PEEP group [32]. The primary outcome was the size of atelectasis detected in the lung CT taken just after operation. Compared with PEEP 4 cmH2O, individualized PEEP patients showed less postoperative atelectasis (6.2 ± 4.1% vs. 10.8 ± 7.1% of lung tissue mass; P = 0.017). Interestingly, this beneficial effects of individualized PEEP for the reduction of driving pressure and atelectasis were more prominent in laparoscopy compared to open surgery [32].
In Park et al. [21], conventional protective ventilation was compared with driving pressure-guided ventilation in thoracic surgery (n = 312). In the driving pressure-guided ventilation, PEEP was titrated to deliver the lowest driving pressure in each patient and applied during one lung ventilation. The PEEP of control group was fixed at 5 cmH2O. The incidence of postoperative pulmonary complications measured by Melbourne scale was 12.2% with conventional protective ventilation, and 5.5% with driving pressure-guided ventilation (OR 0.42, P = 0.047) [21]. The mean difference of driving pressure was just 1 cmH2O [median (IQR), 10 (9 to 11) vs. 9 (8 to 10), P < 0.001] as shown in the previous study of ARDS patients [24]. The authors suggested that the application of individualized PEEP and lower number of patients who showed high driving pressure (> 15 cmH2O, 1/145 vs. 9/147 patients) as the reasons of better outcomes in driving pressure-guided ventilation group.
According to these few prospective studies, PEEP titration can reduce driving pressure and has a possibility as a more advanced ventilation technique.
VT titration
Usually, reduction of VT would decrease driving pressure, but only until the point where reduction of VT does not bring alveolar collapse. In some instances, increased VT was associated with reduction of driving pressure and pulmonary complications [12]. This would probably result from reduction of atelectasis, especially when the level of PEEP is inappropriate [12].
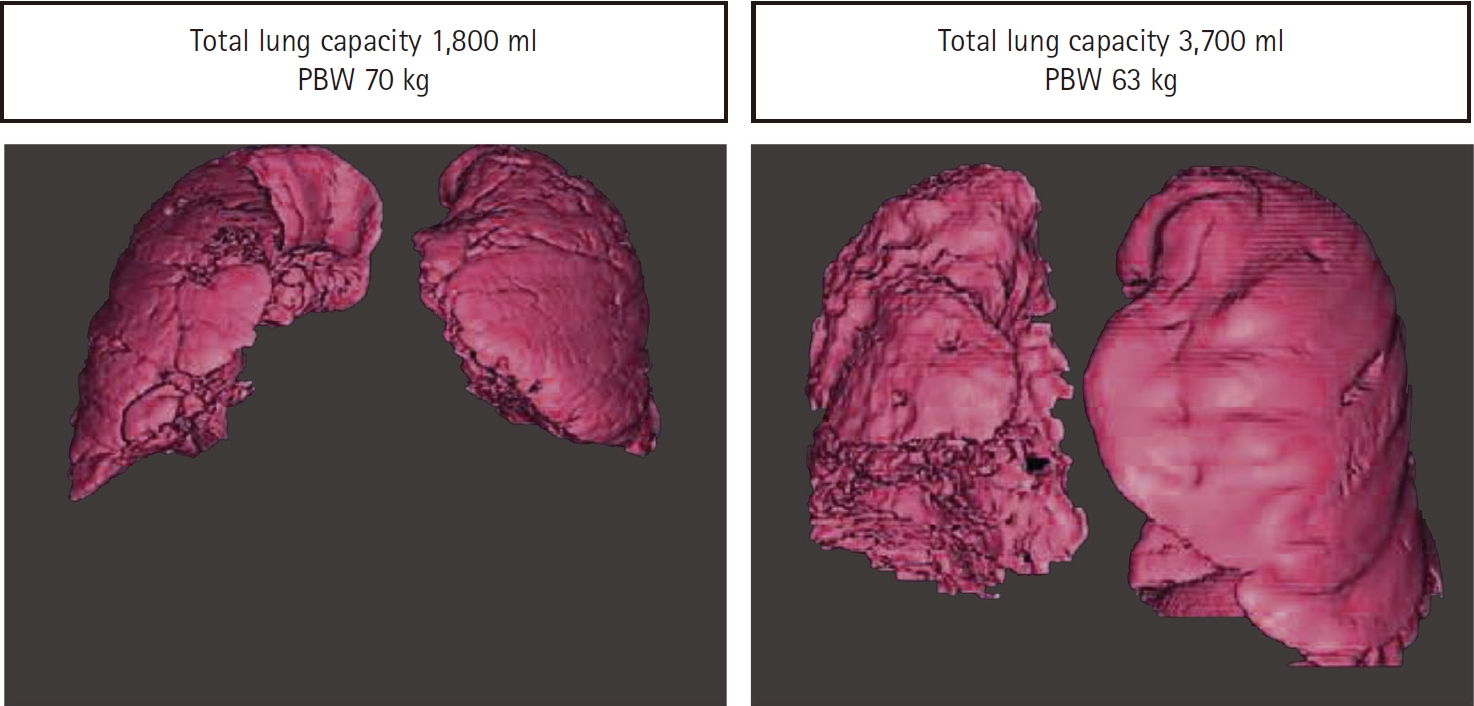
According to 3D lung CT scans, lung size calculation based on the patient’s PBW showed discordance with actual lung size frequently. The correlation between actual lung capacity and PBW based on patient’s height was not strong (R = 0.58-0.65), and was especially poor in patents with low lung compliance (Fig. 4) [37]. If PBW does not reflect patients’ actual lung size reliably, and patients have different ‘functional lung size’ due to underlying lung pathologies, applying fixed VT based on PBW would frequently result in alveolar over-distension or atelectasis. Therefore, additional VT titration guided by driving pressure may be beneficial. However, no randomized studies are available currently.
Alveolar recruitment
Alveolar recruitment is frequently employed to open up the collapsed alveoli before initiation of mechanical ventilation because high PEEP alone is not enough [33,34,38]; When the PEEP increased from 9 to 16 cmH2O, only 47% of patients showed recruitment, but 53% of patients did not. Oxygenation did not improve and static lung elasticity significantly increased in these patients [39]. Recruitment is also essential before starting PEEP titration for driving pressure measurement. However, we do not know how to recruit lungs with various functional sizes.
These days, the ‘open lung’ approach is gaining popularity as a recruitment technique. The open lung approach is to achieve high levels of lung aeration by first conducting recruitment maneuvers to reverse atelectasis and then applying high levels of PEEP to keep recruited alveoli open [40-46]. Recent open lung approaches use a stepwise increase in inspiratory pressure or VT for the recruitment (inspiratory pressure up to 35 - 60 cmH2O, driving pressure up to 20 cmH2O, or VT up to the ventilator limit), and then decremental PEEP titration is performed using volume-controlled ventilation until the highest Cstat is found. The chosen PEEP is usually applied after secondary recruitment (Fig. 5) [40,42,44,45].
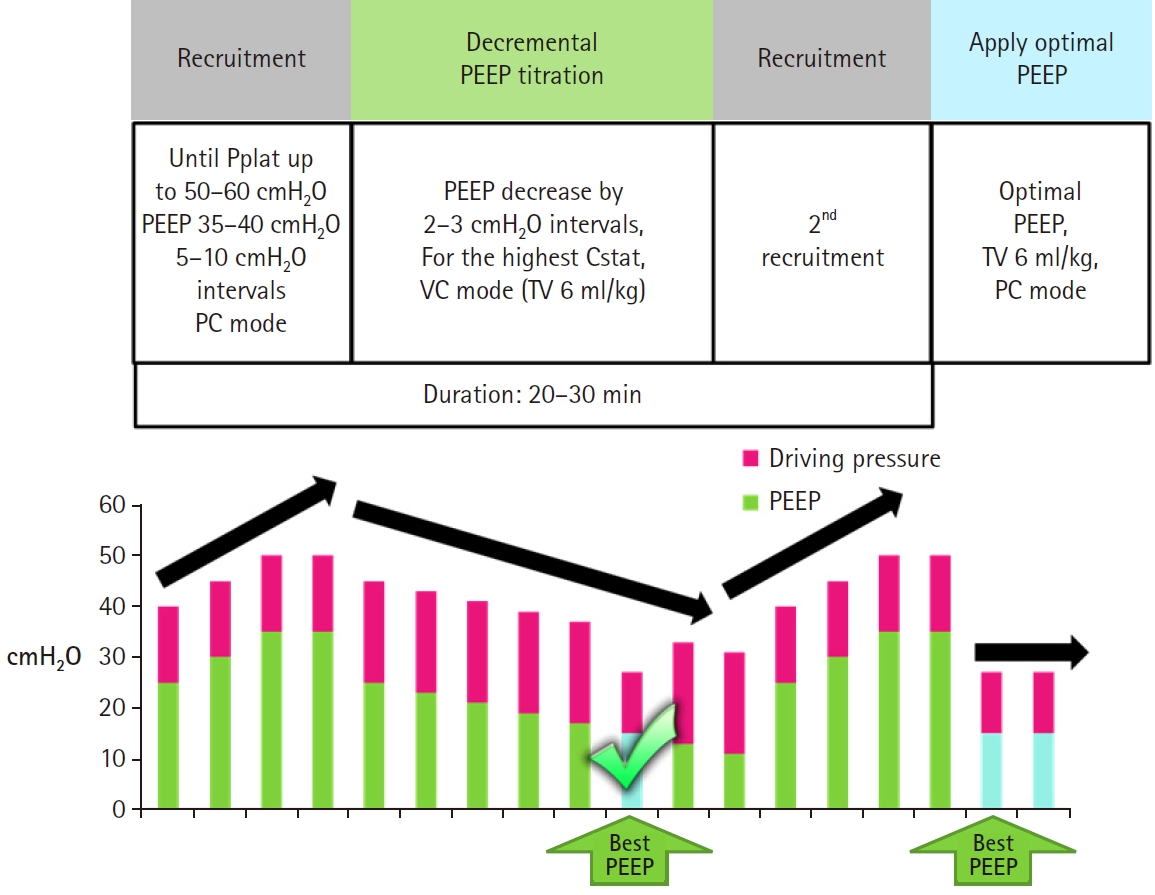
Representative method of the open lung approach. High pressure stepwise recruitment and decremental PEEP titration is performed. Pplat: plateau pressure, PEEP: positive end-expiratory pressure, TV: tidal volume, VC: volume controlled. PC: pressure controlled, Cstat: static lung compliance, Pr: pressure.
However, the open lung approach is not showing consistent results in ICU and surgery. When the open lung approach (recruitment: inspiratory pressure 45 cmH2O + PEEP 30 cmH2O, maintenance PEEP: 13 cmH2O) was compared with a control group (recruitment: inspiratory pressure 20 cmH2O, maintenance PEEP: 8 cmH2O) for ICU patients who showed respiratory insufficiency after open heart surgery, pulmonary severity score was lower (score 2.1 vs. 1.8, P = 0.003, OR 1.86), hospital stay (12 vs. 11 days, P = 0.04) and ICU stays (5 vs. 4 days, P = 0.01) were shorter in open lung approach with no difference in in-hospital mortality (n = 320) [47]. Other large scale randomized studies conducted for ARDS patients showed no improvement in clinical outcomes with the open lung approach compared to regular ARDSnet protocol (n = 200, n = 983) [41,48]. Only lung compliance was increased [41] and the incidence of hypoxia was reduced with open lung approach [41,48]. The latest and largest randomized trial called ART showed worse outcomes with the open lung approach compared to an ordinary ARDSnet protocol (n = 1,200) [42]. In this study, the open lung approach was associated with a higher 28-day mortality, 6-month mortality, and fewer ventilator-free days. This poor outcome seems to be related to barotrauma and hemodynamic instability induced by the open lung approach [42].
Besides the above-mentioned ARDS patients, several studies have been published for surgical patients. For abdominal surgery (PROVHILO trial, n = 900), an open lung approach group (recruitment: inspiratory pressure 35 cmH2O, maintenance PEEP: 13 cmH2O) was compared to a low PEEP group (≤ 2 cmH2O) without recruitment [49]. In the open lung approach group, lung compliance improved, but the incidence of hypotension and the use of vasopressors were higher. Postoperative pulmonary complications, the primary outcome, were reported in 40% and 39% in the open lung approach group and low PEEP group, respectively (relative risk 1.01; 95% CI 0.86–1.20, P = 0.86) [49].
For obese patients (PROBESE trial, n = 2,013, BMI ≥ 35), the open lung approach with high PEEP (recruitment: Pplat 40–50 cmH2O, maintenance PEEP: 12 cmH2O) was compared to low PEEP (4 cmH2O) without recruitment in non-cardiac, non-neurological surgery under general anesthesia. Fewer patients showed hypoxemia (SpO2 < 92%) with the open lung approach [5.0% vs. 13.6%, risk reduction −8.6% (95% CI, −11.1% to 6.1%); P < 0.001], but pulmonary complications were not different [21.3% in the high PEEP group, 23.6% in the low PEEP group; risk ratio, 0.93 (95% CI, 0.83 to 1.04); P = 0.23] [40].
For thoracic surgery, the open lung approach was performed before and after one lung ventilation with the inspiratory pressure 40 and PEEP 20 cmH2O for recruitment. The maintenance PEEP was 8 cmH2O in both the open lung approach and the control groups. The primary outcome was only dead space and PaO2, which were improved with the open lung approach (n = 40) [50].
Overall, the open lung approach seems to improve oxygenation but the beneficial effect on clinical outcome is not certain. Barotrauma may be the main harm of the open lung approach. Barotrauma is an important risk in thoracic surgery and use of recruitment maneuvering at high pressures can cause tension pneumothorax especially in thoracic surgery [51–53].
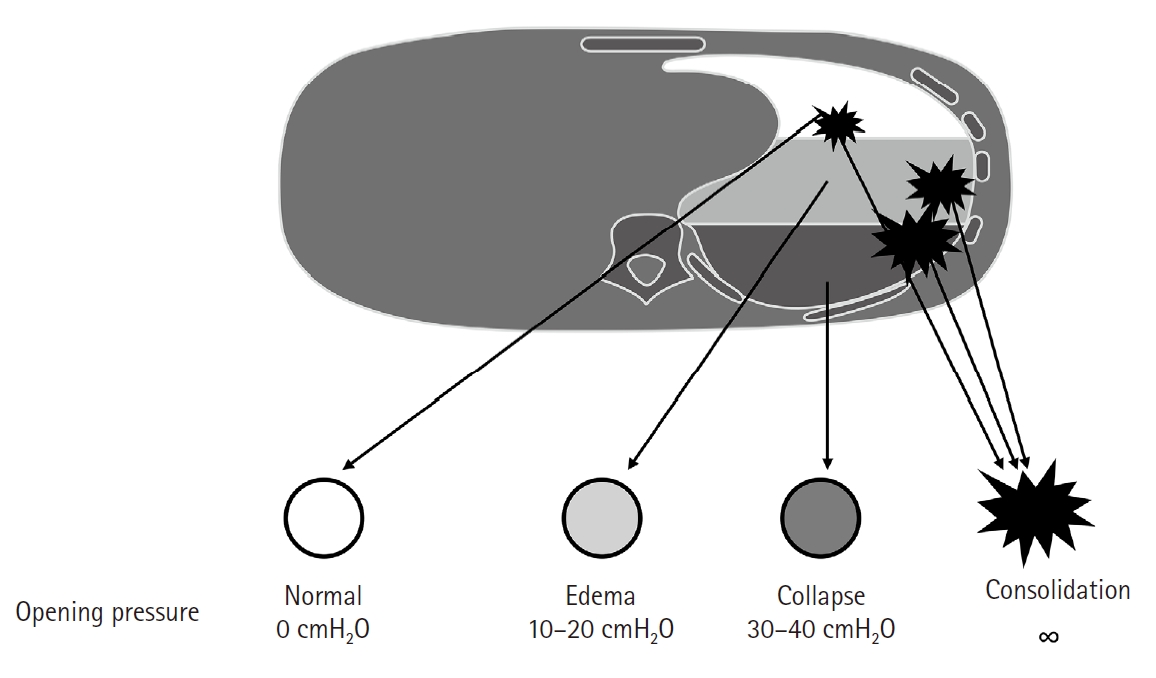
Opening pressures of normal and collapsed alveoli are known as 0 and 30–40 cmH2O, respectively, and consolidated alveoli never open with even higher pressures (Fig. 6) [38]. Non- recruited portion was almost 24% with open lung approach according to the whole-lung CT in ARDS [54]. Healthy alveoli may be damaged during forceful recruitment of collapsed/consolidated alveoli [25]. In an animal study, large VT ventilation recruited more alveoli than small VT ventilation during one lung ventilation, but produced more atelectatic alveoli after the finish of one lung ventilation [55]. Patients who showed a higher percentage of lung recruitment with open lung approach had poorer oxygenation and respiratory-system compliance, and higher rates of death than patients who showed a lower percentage of lung recruitment in ARDS [54]. This may indicate that effective recruitment with the open lung approach only reflects an underlying poor lung condition, but does not necessarily result in improved outcomes.
We do not aim for high oxygenation during ventilation. Instead, we aim for lung protection with acceptable oxygen delivery to tissues. We do not aim for reopening of collapsed/consolidated alveoli at the cost of healthy alveoli. It may be more protective to allow part of the lung to stay closed with permissive atelectasis than to use aggressive effort to keep the lung open [56].
Therefore, regarding recruitment before PEEP titration, we still do not know the best technique for patients with various functional lung sizes. Moderate alveolar recruitment limiting inspiratory pressure < 30 cmH2O or even no recruitment may provide more benefit than the open lung approach [42], but no relevant studies are published yet.
Application of driving pressure-guided ventilation
For driving pressure-guided ventilation, we (the Samsung medical center) usually performs recruitment and PEEP titration as follows. First, recruitment is performed by increasing PEEP from 5 up to 15 cmH2O by 5 cmH2O intervals. Each PEEP level is maintained for 4–5 respiratory cycles (requires < 90 s). During recruitment, respiratory rate is 10 /min, inspiratory:expiratory duration = 1:1, inspiratory pause 30%, VT 8 ml/kg PBW for two lung ventilation, 5 ml/kg PBW for one lung ventilation. Recruitment is stopped if Pplat reaches 30 cmH2O. The second step is PEEP titration. PEEP starts at 10 cmH2O and is then decreased to 0 cmH2O by 1–2 cmH2O intervals. Driving pressure is measured at each PEEP level after maintaining for 5 respiratory cycles. The PEEP which shows the lowest driving pressure is determined. If multiple levels of PEEP show the same lowest driving pressure, the lowest PEEP is chosen. During PEEP titration, respiratory rate is 12 /min, inspiratory:expiratory duration = 1:2, inspiratory pause 30%, VT 8 ml/kg PBW for two lung ventilation, 5 ml/kg PBW for one lung ventilation (requires < 150–275 s). The chosen PEEP is applied throughout the ventilation. Additional recruitment and PEEP titration is performed when driving pressure increases by 2 cmH2O from the baseline, or when the ventilator setting is changed. The optional step is VT titration. If driving pressure is still higher than 15 cmH2O, VT is decreased by 1 ml/kg PBW until driving pressure falls below 15 cmH2O (VT down to 6 ml/kg PBW for two lung ventilation and to 3 ml/kg PBW for one lung ventilation). If driving pressure increases with VT reduction, VT is increased by 1 ml/kg PBW until driving pressure falls below 15 cmH2O (VT up to 10 ml/kg PBW for two lung ventilation and to 7 ml/kg PBW for one lung ventilation). Usually, recruitment and PEEP titration finish within 5 minutes and VT titration is not required. If driving pressure is maintained higher than 15 cmH2O with either method, we expect high postoperative pulmonary complications and prepare more advanced postoperative care.
Currently undergoing large randomized studies for thoracic surgery
PROTHOR
In this study, an open lung approach with maintenance PEEP 10 cmH2O is being compared to PEEP 5 cmH2O without recruitment (n = 2,378) [43].
iPROVE-OLV
In this study, PEEP 5 cmH2O without recruitment is compared to the open lung approach with individualized PEEP based on Cstat (n = 1,380) [44]. The same group used the same protocol for abdominal surgery but failed to show a difference between the two ventilation techniques (iPROVE trial, n = 1,012, relative risk 0.74 to 1.07) [45]. In the previous abdominal surgery study, they performed 4 group comparisons, resulting in an underpowered study. They changed their protocol to a comparison of two groups for the iPROVE-OLV trial [44]. In this trial, individualized high flow O2 or fixed O2 supply at post-anesthesia care unit is also included in the protocol.
These studies using individualized PEEP based on Cstat are currently underway. It would be a very important finding if this ventilatory strategy proves to be effective and brings improved outcomes. However, there is a concern regarding the use of a high-pressure open lung approach.
Conclusion
Driving pressure guided ventilation might be another technique to reduce postoperative pulmonary complications and improve recovery in thoracic and general surgery patients. However, there are not many studies on this topic yet. Thus, more prospective, randomized trials are requested to assess the independent role of driving pressure and PEEP titration for clinical outcomes. VT titration based on driving pressure would also warrant further study.
Notes
No potential conflict of interest relevant to this article was reported.
Author Contributions
Hyun-joo Ahn (Conceptualization; Data curation; Formal analysis; Investigation; Supervision; Validation; Writing – original draft; Writing – review & editing)
MiHye Park (Conceptualization; Data curation; Formal analysis; Investigation; Writing – original draft; Writing – review & editing)
Jie Ae Kim (Writing – original draft; Writing – review & editing)
Mikyung Yang (Writing – original draft; Writing – review & editing)
Susie Yoon (Conceptualization; Writing – original draft; Writing – review & editing)
Bo Rim Kim (Conceptualization; Writing – original draft; Writing – review & editing)
Jae-Hyon Bahk (Conceptualization; Writing – original draft; Writing – review & editing)
Young Jun Oh (Conceptualization; Writing – original draft; Writing – review & editing)
Eun-Ho Lee (Conceptualization; Writing – original draft; Writing – review & editing)