Surgical site infection after colorectal surgery according to the main anesthetic agent: a retrospective comparison between volatile anesthetics and propofol
Article information
Abstract
Background
Anesthetic agents used for general anesthesia are emerging possible influential factors for surgical site infection (SSI). In this retrospective study, we evaluated the incidence of SSI after colorectal surgery according to the main anesthetic agents: volatile anesthetics vs. propofol.
Methods
A total 1,934 adult patients, who underwent elective colorectal surgery under general anesthesia between January 2011 and December 2013, were surveyed to evaluate the incidence of SSI: 1,519 using volatile anesthetics and 415 using propofol for main anesthetic agents. Patient, surgery, and anesthesia-related factors were investigated from all patients. Propensity-score matching was performed to reduce the risk of confounding and produced 390 patients in each group.
Results
Within the propensity-score matched groups, the incidence of SSI was higher in the volatile group compared with the propofol group (10 [2.6%] vs. 2 [0.5%], OR = 5.0 [95% CI = 1.1-2.8]). C-reactive protein was higher in the volatile group than in the propofol group (8.4 ± 5.6 vs. 7.1 ± 5.3 mg/dl, P = 0.001), and postoperative white blood cells count was higher in the volatile group than in the propofol group (9.2 ± 3.2 × 103/µl vs. 8.6 ± 3.4 × 103/µl, P = 0.041).
Conclusions
The results of this study suggest that intravenous anesthesia may have beneficial effects for reducing SSI in colorectal surgery compared to volatile anesthesia.
Introduction
Surgical site infection (SSI) is the most common postoperative major complication followed by respiratory, thromboembolic, and cardiovascular complications, and it is one of the predictors of mortality and morbidity after surgery [1]. SSI interferes with wound healing, which increases the postoperative morbidity, hospital stay, and medical cost [2]. Surgical or patient-related conditions have generally been considered to be contributing factors to SSI; however, it has become more probable that perioperative anesthetic care would influence SSI [34]. Careful anesthetic and perioperative managements, such as body temperature [5], intravascular volume status [6], inspired oxygen concentration [7], and blood transfusion [8], were required for the prevention of SSI. Considering the anesthetic technique, regional anesthesia was reported to have beneficial effects for SSI or systemic infection compared with general anesthesia [910].
Anesthetic agents used for general anesthesia are also becoming influential factors for SSI. Opioid administered for the management of intra- and postoperative pain showed immunosuppressive effects [11]. In addition, remifentanil-based general anesthesia was reported to increase the postoperative SSI rate [12]. On the contrary, it was revealed that propofol had anti-inflammatory and anti-oxidative effect at clinical plasma concentrations [13]. Moreover, alveolar macrophage was reported to express less proinflammatory cytokine genes during propofol anesthesia than isoflurane anesthesia [14].
Despite the differential effects of anesthetic agents on the immune or inflammatory response, the incidence of SSI has rarely been studied in specific clinical conditions. Under the hypothesis that patients managed with intravenous anesthesia would have less SSI compared with those receiving volatile anesthetic agent, we retrospectively evaluated the incidence of SSI after colorectal surgery under general anesthesia by different main anesthetic agents: volatile anesthetics vs. propofol.
Materials and Methods
Study population and data source
After getting an approval from the Institutional Review Board, we retrospectively reviewed the electronic medical records of all adult inpatients who underwent elective colorectal surgery under general anesthesia from January 2011 to December 2013. The following ICD-9-CM procedure codes were used to select appropriate patients: 45.7, 45.8, 46.1, 46.52, 48.5, 48.63, and 17.3. This study was exempted from the need to obtain informed consent and was registered at ClinicaTrials.
The independent variable of interest was the main anesthetic agents used for general anesthesia, and the primary study outcome was whether there was an SSI occurrence or not during the postoperative 30-day period. The occurrence of SSI included any superficial-incisional, deep-incisional, and organ/space SSIs. In our hospital, a patient suspected of having SSI was primarily examined by a surgeon and, if necessary, an infection specialist. SSI was finally diagnosed according to the criteria of the Centers for Disease Control and Prevention (Appendix) [15] and reported to the Infection Control Department of our hospital by a web-based recording system.
General anesthesia technique
General anesthesia and patient care were generally performed as follows. At the preoperative reception area, midazolam 1.5–2 mg was administered intravenously to patients in order to relieve anxiety. After arriving at the operating room, non-invasive arterial pressure, pulse oximetry, and electrocardiogram were routinely monitored before the induction of general anesthesia. The main anesthetic technique was either volatile (volatile group) or intravenous anesthesia (propofol group) according to the preference of the attending anesthesiologists. For the volatile group, a bolus dose of 1.5–2 mg/kg propofol was administered to patients for the induction of general anesthesia, and then sevoflurane or desflurane for the maintenance of anesthesia. For the propofol group, propofol was given to patients via a target-controlled infusion (TCI) device (Orchestra; Fresenius Vial, Brezins, France) as the main anesthetic drug. The effect site concentration (Ce) of propofol was titrated during the operation on the basis of bispectral index. Remifentanil was given via TCI in both volatile and propofol groups for intraoperative analgesia. The Ce of remifentanil was usually titrated depending on the autonomic response, such as arterial pressure or heart rate, during the operation. Rocuronium was used for tracheal intubation as well as intraoperative muscle relaxation. Patient's body temperature was monitored via the esophageal probe. To maintain body temperature, a circulating water-heating pad was placed on the operating table and a forced-air warmer was applied on the upper body of patients during the operation. Intravenous prophylactic antibiotic agent was given to each patient within 30 min before surgical incision.
Outcome variables
For the evaluation of SSI occurrence, we collected the following data related to SSI: (1) Patient-related factors like basic characteristics of patients including age, gender, height, weight, body mass index (BMI), preoperative blood glucose level, American Society of Anesthesiologists (ASA) physical status, current smoking state, and the lowest body temperature during the operation; (2) Surgery-related factors including the operator name, the operation name, operation type (laparotomy or laparoscopy), the duration of operation, and location where patients were discharged after finishing the operation; (3) Anesthesia-related factors including the main anesthetic agents, duration of anesthesia, use of postoperative patient-controlled analgesia, and perioperative blood transfusion; and (4) blood tests including pre- and postoperative white blood cell (WBC) count with the percentage of segmented neutrophils and postoperative C-reactive protein (CRP). Preoperative blood tests were chosen within 3 months prior to the operation. Postoperative blood tests were performed at postoperative first day.
Statistics
Student's t-test or chi-square test including odds ratio (OR) and 95% confidence interval (CI) was conducted to examine the differences between the volatile and the propofol groups. A binary logistic regression model was applied to determine whether or not there were predisposing factors in the occurrence of SSI, and the strength of association is presented as the OR with 95% CI. The dependent variable was the occurrence of SSI. The independent variables included age, BMI, ASA physical status, operator, operation time, general anesthesia technique, laparotomy or laparoscopic surgical procedure, current smoking status, glucose range, and perioperative transfusion.
Propensity score matching was performed to reduce the risk of confounding between the volatile and the propofol groups. Propensity scores were calculated by logistic regression model. The independent variables were age, gender, BMI, ASA, operator, operation type, preoperative smoking, glucose level, and perioperative transfusion. The dependent variable was the main anesthetic technique: volatile or intravenous anesthesia. We performed nearest-neighbor matching with caliper of 0.1. We computed and compared the absolute standardized differences between the two groups. For propensity score matched data, paired t-test or McNemar test was performed appropriately. In addition, conditional logistic regression analysis with one covariate, anesthetic type, was used to calculate the OR and 95% CI for occurrence of SSI.
All analyses were performed by using R software program (ver. 3.1.2; R Foundation for Statistical Computing, Vienna, Austria) and SPSS software (ver. 22; IBM Corp., Armonk, NY, USA), and a P value of less than 0.05 was considered statistically significant.
Results
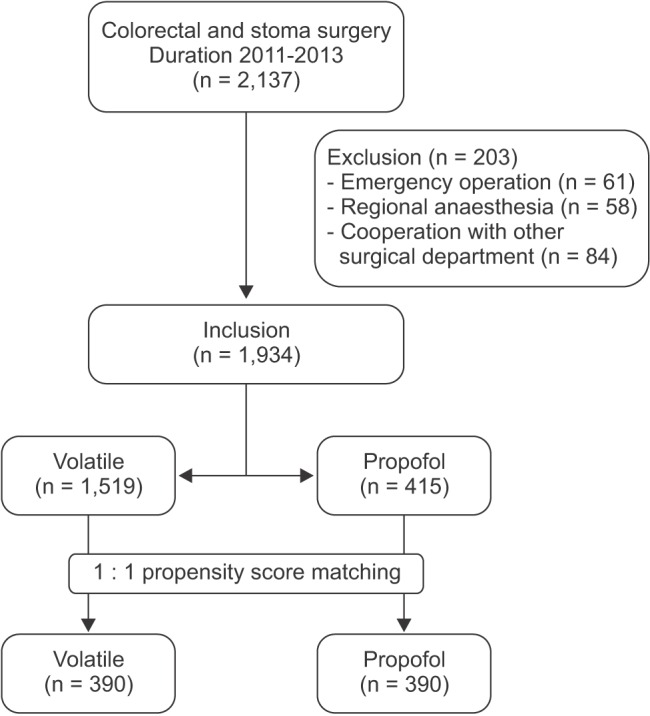
Total 2,137 inpatient-records were evaluated and 203 patients were excluded from the study due to the emergency operation (n = 61), regional anesthesia (n = 58), and cooperation with other surgical department (n = 84). Of the remaining 1,934 records, volatile and intravenous anesthesia were performed in 1,519 and 415 patients, respectively, and 390 patients were selected in each group by the propensity score matching process (Fig. 1).
The patients' characteristics and the information regarding the surgery and anesthesia are presented in Table 1, and significant differences were found in the name of operation between the two groups. Stoma operations were done significantly more frequently in the propofol group than in the volatile group (P < 0.001). In the propensity score-matched cohorts, this different parameter became comparable between the two groups.
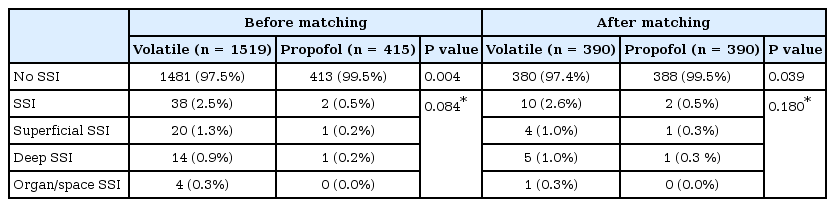
The overall incidence of SSI was higher in the volatile group than in the propofol group (38 [2.5%] vs. 2 [0.5%], OR = 5.3 [95% CI = 1.3–22.1, P = 0.004). In the propensity score-matched cohorts, SSI also occurred more frequently in the volatile group than in the propofol group (10 [2.6%] vs. 2 [0.5%], OR = 5.0, 95% CI = 1.1–22.8, P = 0.039). However, when we subdivided the class of SSI, the incidence was not different between the two groups before and after the propensity matching (P = 0.084 and 0.180, respectively) (Table 2).
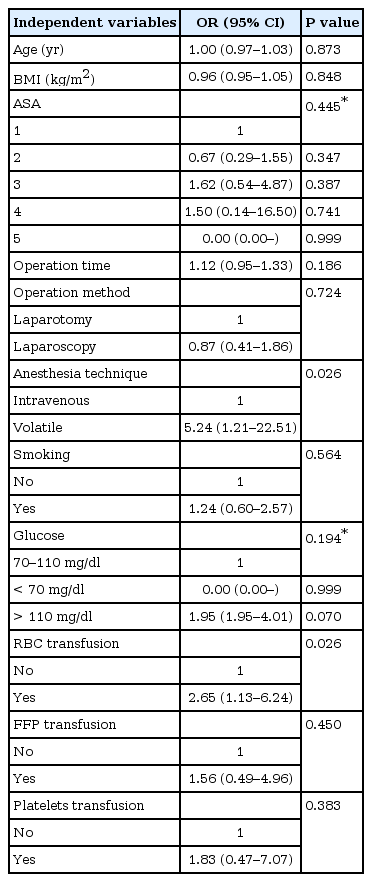
Table 3 showed the results of logistic regression analysis. Factors found to be significantly influential for the occurrence of SSI were the followings: anesthetic technique, with the volatile group being 5.3 (95% CI, 1.2–22.5; P = 0.026) times more likely to experience SSI than the propofol group; perioperative red blood cells (RBC) transfusion, with patients receiving RBC being 2.7 (95% CI, 1.1–6.2; P = 0.026) times more likely to experience SSI than patients not receiving RBC.
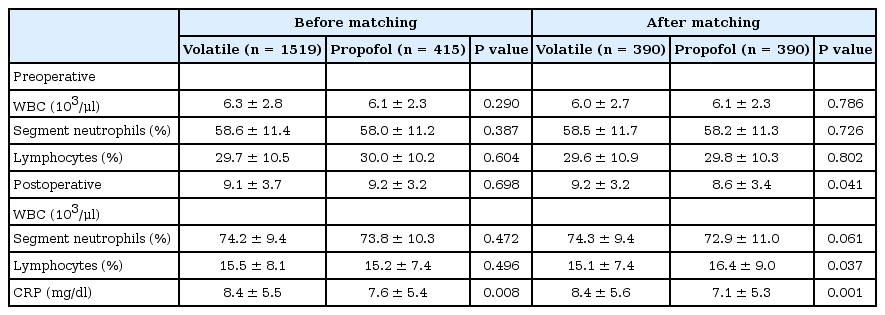
Preoperative WBC count and the portion of segment neutrophils and lymphocytes were comparable between the two groups before and after propensity matching. Postoperative WBC count was lower and the portion of lymphocytes was higher in the propofol group after the propensity matching. Irrespective of the propensity score matching, postoperative CRP was higher in the volatile group than in the propofol group (Table 4). When our patients were subdivided into the SSI group (n = 40) or non-SSI group (n = 1,894), CRP of the SSI group were significantly higher than that of the non-SSI group (9.7 ± 6.2 mg/dl in SSI group vs. 7.7 ± 5.4 mg/dl in non-SSI group, P = 0.021). The proportion of patients who received blood transfusion during the perioperative period was not different between the volatile and the propofol groups (Table 5).
Discussion
In this study, we found that SSI occurred less frequently in patients undergoing elective colorectal surgery receiving intravenous anesthesia compared to volatile anesthesia.
With regard to the anesthetic agents, propofol was known to have anti-inflammatory and anti-oxidant activity. Propofol reduced the production of proinflammatory cytokines or chemokines, such as tumour necrosis factor-α, interleukin (IL)-1, IL-6, and IL-8 [161718], and it inhibited the production of reactive oxygen species (ROS) and decreased the oxidative stress [1920]. Enhanced ROS generation plays a key role in the progression of inflammatory disease [21], and previous studies reported that inhibition of overproduction of ROS could improve the inflammatory reaction [22].
Conversely, it was reported that macrophage aggregation and neutrophil influx were more pronounced in inhalation anesthesia than propofol anesthesia [23], and gene expression of proinflammatory cytokines was increased after inhalation anesthesia [24]. In addition, volatile anesthetics modulated the p38 mitogen-activated protein kinase [25], which is a significant signaling pathway to up-regulate the cytokine production in inflammation [26].
This discrepancy between the type of main anesthetic agents seems to impact the SSI occurrence in our patients. Although there have been various results analysing the effect of each anesthetic agent on the inflammatory biomarker or immunomodulation, the incidence of SSI has rarely been investigated according to the main anesthetic agent. To the best our knowledge, only one study evaluated the result of SSI according to different general anesthetic agents. In contrast to our results, Shimizu et al. [27], in a retrospective study, reported that SSI was less likely to occur after sevoflurane anesthesia in elective open gastrointestinal surgeries compared with propofol anesthesia. However, an important drawback in their study was that they provided either epidural injection of ropivacaine or continuous intravenous infusion of remifentanil for intraoperative analgesia to each patient, which was not considered for the analysis of postoperative SSI. Some studies have already reported that regional anesthesia is related to a reduced occurrence of SSI as well as less systemic infectious complications than general anesthesia in some clinical settings [910]. Moreover, opioids, such as morphine and fentanyl, were known to result in immunosuppression [11], and it was also reported that remifentanil-based anesthesia increased the incidence of SSI compared with fentanyl-based anesthesia [12]. Our patients in both groups were given intraoperative remifentanil in common, thus, there did not seem to be confounding factors related to the intra- and postoperative analgesic method in our cohort. However, it is still apprehended whether there was any difference in the amount of remifentanil infused during the operation, which could not be verified retrospectively in the anesthetic records.
CRP is found in blood plasma, the level of which increases in response to infection/inflammation. The propofol group patients showed lower CRP level irrespective of the propensity-score matching, thus, they might have less acute inflammatory reaction at postoperative 1 day. CRP was already known to be related with postoperative infectious complications [28].
Several limitations should be emphasized in this study. First, the OR for SSI would change enormously, if a couple more SSI occurred in the propofol group by chance. Along this line, an OR of 5 is more or less high, even if anesthetic agents influence the occurrence of SSI. Second, due to the retrospective nature of this study, several risk factors, such as surgical suture quality, mechanical tension on the wound, tissue perfusion, quality of analgesia, perioperative volume status, and inspired fraction of oxygen, could not be evaluated properly. These variables could affect the outcome although we used the propensity score matching to minimize the selection bias. Third, patients of the volatile group received 1.5–2.5 mg/kg of propofol for inducing general anesthesia. Even though propofol is metabolized rapidly in the liver, we could not thoroughly exclude the potential effect of a single dose of propofol on SSI in the volatile group. Last, the volatile group included all patients received any volatile anesthetic, sevoflurane or desflurane, for the main anesthetic agent. However, sevoflurane has been reported to have more favorable effect on anti-inflammatory responses [2930]. Definite clinical outcome has not been turned out according to the sort of volatile anesthetics, and a further study is required to address this issue.
In summary, our results suggest that intravenous anesthesia has beneficial effects for reducing SSI in colorectal surgery compared with volatile anesthesia. A larger prospective study incorporating a standardized anesthesia protocol is now required to further investigate the role of different general anesthesia techniques in postoperative SSI.
Acknowledgments
The authors thank the Medical Research Collaborating Center at Seoul National University Bundang Hospital for statistical analyses.
Appendices
Appendix
Diagnosis Criteria for Surgical Site Infection (SSI)