The effects of intravenous dexmedetomidine on spinal anesthesia: comparision of different dose of dexmedetomidine
Article information
Abstract
Background
In this prospective, randomized, double-blind, placebo-controlled trial, we tried to find out appropriate amounts of single-dose dexmedetomidine to prolong the duration of spinal anesthesia in a clinical setting.
Methods
Sixty patients who were scheduled for unilateral lower limb surgery under spinal anesthesia were randomized into three groups receiving normal saline (control group, n = 20) or 0.5 or 1.0 ug/kg dexmedetomidine (D-0.5 group, n = 20; D-1, n = 20) intravenously prior to spinal anesthesia with 12 mg of bupivacaine. The two-dermatome pinprick sensory regression time, duration of the motor block, Ramsay sedation score (RSS), and side effects of dexmedetomidine were assessed.
Results
The two-dermatome pinprick sensory regression time (57.6 ± 23.2 vs 86.5 ± 24.3 vs 92.5 ± 30.7, P = 0.0002) and duration of the motor block (98.8 ± 34.1 vs 132.9 ± 43.4 vs 130.4 ± 50.4, P = 0.0261) were significantly increased in the D-0.5 and D-1 groups than in the control group. The RSS were significantly higher in the D-0.5 and D-1 groups than in the control group. However, there were no patients with oxygen desaturation in dexmedetomidine groups. The incidences of hypotension and bradycardia showed no differences among the three groups.
Conclusions
Both 0.5 and 1.0 ug/kg of dexmedetomidine administered as isolated boluses in the absence of maintenance infusions prolonged the duration of spinal anesthesia.
Introduction
Dexmedetomidine is a highly selective α2-adrenergic agonist that provides analgesic and anesthetic-sparing effects [1,2]. In addition, because it affects the locus ceruleus area, which is associated with the modulation of sleep and respiration, it has a sedative effect with minimal respiratory depression [3,4]. In previous studies, intravenous administration of dexmedetomidine before or after spinal anesthesia prolonged the duration of sensory and motor block [5,6,7,8,9]. Commonly used intravenous methods of dexmedetomidine include a single-dose intravenous administration [5,6,7] before or after spinal anesthesia and a loading dose followed by continuous infusion [8,9]. However, a loading dose followed by continuous infusion has been reported to increase the incidence of hypotension and bradycardia [9,10]. A number of clinical studies have reported the effects of single-dose intravenous dexmedetomidine on spinal anesthesia with various amounts, ranging from 0.25 to 1 µg/kg [5,6,7,8,9,10]. In general, these studies have compared a control group and an experimental group in which patients are administered only one fixed amount of dexmedetomidine [6,8,9,10]. A few studies have directly compared different amounts of dexmedetomidine.
Therefore, we evaluated whether different amounts (0.5 µg/kg and 1.0 µg/kg) of single-dose intravenous dexmedetomidine before spinal anesthesia would cause different durations of spinal anesthesia. We also evaluated sedation scores, and dexmedetomidine-related side effects. Through this double blind, randomized placebo-controlled clinical study, we tried to find out the appropriate amounts of single-dose dexmedetomidine for prolongation duration of spinal anesthesia in a clinical setting.
Materials and Methods
After receiving approval from the Institutional Review Board of our hospital, 60 adult patients who were scheduled for surgery under spinal anesthesia were enrolled in this study. Written informed consent was obtained in all cases. All subjects had an American Society of Anesthesiologists physical status classification of either I or II, and all were between the ages of 18 and 65 years. This study was conducted from July 2013 to January 2014. Patients were excluded from this study if they had contraindications to regional anesthesia, including coagulopathy, or local skin infection, uncontrolled hypertension, diabetes, cardiopulmonary disease, and/or a body mass index of less than 18.5 or greater than 30 kg/m2. Nursing staff blind to the purposes of the study randomly allocated patients to one of three groups (the control, the D-0.5, and the D-1 group) using a computergenerated randomization table.
Because the supine position should be maintained and at least one leg should be left free to test motor function during surgery, patients who were scheduled for unilateral lower limb surgery under spinal anesthesia were preferred. One anesthesiologist took charge of preparing the study drug before the patients arrived in the operating room. The prepared solution was a mixture of normal saline (20 µg/ml of dexmedetomidine, a total volume of 10 ml within a 20 ml syringe). We used dosages of 0.5 µg/kg and 1.0 µg/kg of dexmedetomidine (Precedex®; Hospira, Rocky Mount, NC, USA, 200 µg/2 ml) for the D-0.5 and D-1 groups, respectively, and only normal saline for the control group. There were no labels on the syringes. After the preparation of the drug, another anesthesiologist blind to the patients' groups took charge of the induction and monitoring of anesthesia during the surgery.
None of the patients received premedication. Upon arrival in the operating room, standard monitoring devices including an electrocardiogram, a pulse oximeter, and a noninvasive blood pressure cuff were applied. Before undergoing spinal anesthesia, all patients were administered 500 ml of lactated Ringer's solution for pre-loading, after which the study drug was administered over a 10 min period. The baseline mean arterial pressure (MAP), heart rate (HR), and pulse oxygen saturation (SpO2) were recorded. Five minutes after end of study drug infusion, the patient was placed in the lateral decubitus position. Spinal anesthesia was performed at the midline of the L4-5 interspinous space. After the intradermal infiltration of 3 ml of 2% lidocaine for local anesthesia, a 25-gauge Sprotte needle was used for a lumbar puncture. When a free flow of cerebrospinal fluid was confirmed, 12 mg of 0.5% hyperbaric bupivacaine (Marcaine®, Astra Zeneca, Sweden) was injected into the subarachnoid space for 20 sec. Following the spinal anesthesia, patients were repositioned to the supine position and received 5 L/min of oxygen via a facial mask.
The sensory block level was assessed by testing the loss of pinprick sensation with a blunt 25-guage needle along the midclavicular line bilaterally. The motor block level was assessed according to the Modified Bromage Scale (0 = no paralysis; 1 = unable to raise extended leg; 2 = unable to flex knee; 3 = unable to flex ankle) [11]. The sensory block level and the modified Bromage scale were assessed every 2.5 min within 20 min after the spinal injection and then every 10 min afterwards. The Ramsay sedation score (RSS) was used to assess sedation (1 = anxious and agitated; 2 = cooperative and tranquil; 3 = drowsy but responsive to verbal commands; 4 = asleep but briskly responsive to tactile stimulation; 5 = asleep and sluggish responses to stimuli; and 6 = asleep and no response) [12]. The MAP, HR, and SpO2 levels and the RSS were recorded every 5 min.
Hypotension was defined as below 80% of the baseline mean arterial pressure or below 90 mmHg of systolic blood pressure. If hypotension developed, ephedrine 4 mg was injected intravenously. If the blood pressure drop continued, the same dose was injected repeatedly. Bradycardia was defined as HR < 45 beats/ min and was treated with 0.5 mg of intravenous atropine. Desaturation was defined as a SpO2 level of less than 90% and was treated appropriately.
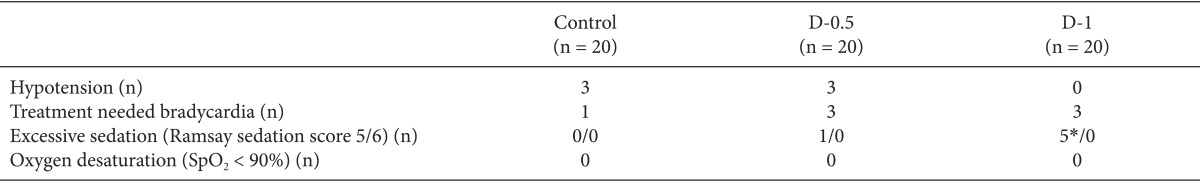
The primary outcome of this study was a comparison of the durations of spinal sensory and motor blocks among the three groups. The duration of the sensory block was defined as a twodermatome regression from the maximal level. Motor block duration was the time required to return to a modified Bromage scale of 1 after the achievement of 3. If the maximal modified Bromage scale didn't approach number 3, motor block duration was defined score 1 after the achievement of 2. The secondary outcomes were an evaluation of the sedation score and the regression time for RSS which is the time required to return to a RSS of less than 3 after the achievement of a score of 3. If the maximal RSS didn't approach number 3, regression time of RSS was defined the time to return to a score 2 or under. We also evaluated the side effects of dexmedetomidine, including bradycardia, hypotension, oxygen desaturation and excessive sedation.
The statistical analysis was performed with SAS (Version 9.2, SAS Inc., Cary, NC, USA). Data were expressed as the mean ± SD, the median (range), or the number of patients. The sample sizes were calculated based on a previous study [5], assuming that the difference in the sensory block duration for pain between the control group and either the D-0.5 or D-1 group was more than 30 min on average, 30 min to the standard deviation, with an alpha error of 0.05 and a power of 80%. A total of 16 patients per group were necessary to be able to demonstrate statistical significance. Therefore, we assigned 20 patients to each group to allow for possible protocol violations during the study period.
To compare the variables between the three groups, the Kolmogorov-Smirnov test was used to identify the variables with a normal distribution. Variables with a normal distribution were compared using an ANOVA test, and those without a normal distribution were compared using the Kruskal-Wallis test. Incidence variables were compared using a chi-square test or Fisher's exact test. All significant results were further analyzed with the Bonferroni post-hoc test. A P value of less than 0.05 was considered to be statistically significant.
Results
Sixty patients were enrolled in this study, and none were excluded or failed to complete (Fig. 1). In total, data from 60 patients were analyzed, with no differences observed in any of the demographic variables (Table 1).
The time for the two-segment regression of the pinprick sensory block was significantly prolonged in the D-0.5 group (86.5 ± 24.3, P = 0.001) and the D-1 group (92.5 ± 30.7, P < 0.0001) compared to the control group (57.6 ± 23.2). The time for regression of the motor block (Bromage scale from 3 to 1) was also significantly prolonged in the D-0.5 group (132.9 ± 43.4, P = 0.0152) and the D-1 group (130.4 ± 50.4, P = 0.024) compared to the control group (98.8 ± 34.1). However, there were no statistically significant differences between the D-0.5 and D-1 groups in the time for two-segment regression of the pinprick sensory block and the time for regression of the motor block (Table 2). The number of patients that maximal modified Bromage scale didn't approach number 3 were 2, 1, and 2 of 20 patients in the control, D-0.5 and D-1 groups, respectively.
Fig. 2 shows the RSS at each time point. The RSS were significantly increased in the dexmedetomidine groups than in the control group after following injection of dexmedetomidine. In addition, in the D-1 groups the RSS were significantly higher than in the D-0.5 group. The excessive sedation (RSS > 4) was observed in 1 of 20 patients and 5 of 20 patients in the D-0.5 and D-1 groups, respectively. However, there were no patients with oxygen desaturation among the three groups (Table 3). The regression time of the RSS (< 3) was 83.6 ± 40.4 and 89.9 ± 42.7 in the D-0.5 and D-1 groups, respectively.

Ramsay sedation score. Values are presented as mean ± SD. *P < 0.05 when compared to control group. †P < 0.05 D-1 group when compared to the value in the D-0.5 group at the same time point.
The median of the maximal pinprick sensory block level and the time to reach the T10 dermatome level were not statistically different among the groups (Table 2). The heart rate during the study was not statistically different among the groups. The incidences of hypotension and bradycardia requiring treatment also showed no differences among the groups.
Discussion
Our results indicate that both 0.5 and 1.0 ug/kg of dexmedetomidine administered as isolated boluses in the absence of maintenance infusions prolonged the duration of sensory and motor block of spinal anesthesia. In dexmedetomidine groups, the sedation score were significantly increased than in the control group, and duration of sedation (regression time of the RSS) was 83.6 ± 40.4 and 89.9 ± 42.7 in the D-0.5 and D-1 groups, respectively. In addition, no patients showed an oxygen desaturation among the three groups.
Several clinical studies have investigated the effects of intravenous dexmedetomidine on spinal anesthesia. Previous studies used various doses and types of local anesthetics for spinal anesthesia as well as various doses and infusion methods of intravenous dexmedetomidine, so it is not easy to reliable translation into clinical practice. Mostly, dexmedetomidine was administered at an initial loading dose from 0.25 to 1 ug/kg, and/or a maintenance infusion with rates between 0.2 and 0.5 ug/kg/h throughout the duration of surgery [5,6,7,8,9,10]. According to the results of previous clinical studies, intravenous dexmedetomidine can prolong the duration of sensory blockade and, to a lesser extent, prolong the motor blockade duration [13]. However, few studies have directly compared different amounts of dexmedetomidine. In particular, 0.5 ug/kg and 1 ug/kg are commonly used doses in clinical practice. In our study, single doses of 0.5 ug/kg and 1 ug/kg of dexmedetomidine prolonged the two-segment regression times of the sensory block and motor block. However, there were no statistically significant differences in the duration of spinal anesthesia between the D-1 and the D-0.5 group. It should be noted that an analgesic ceiling effect of dexmedetomidine was apparent at a dose of 0.5 ug/kg in a previous study [14].
The mechanism of intravenous dexmedetomidine on spinal anesthesia remains unclear; however, supra-spinal, direct analgesia, and/or vasoconstriction activities are involved [15]. Moreover, dexmedetomidine produces a greater degree of differential blockade by preferentially blocking the myelinated A α-fibers involved in sensory conduction over the unmyelinated C fibers involved in motor conduction [13].
The administration of intravenous dexmedetomidine in spinal anesthesia may actually have a dual effect by both enhancing the local anesthetic action and providing sedation. Dexmedetomidine affects the locus caeruleus area of the brain, which induces sedation resembling natural sleep by means of sleep modulation and respiration control [3,16]. It is correlated with cooperative sedation, which is different from the clouding of consciousness that occurs with drugs that act on GABA receptors, such as propofol or midazolam [17]. In a previous study, dexmedetomidine showed better oxygen saturation and RSS than midazolam [18]. In the present study, dexmedetomidine provided sufficient sedation (0 vs 83.6 ± 40.4 vs 89.9 ± 42.7 min), and the duration did not differ between the D-0.5 and D-1 groups. Although the excessive sedation (RSS > 4) was observed in 1 of 20 patients and 5 of 20 patients in the D-0.5 and D-1 groups, respectively, there were no patients with oxygen desaturation.
In spite of its many advantages as a sedative drug, dexmedetomidine causes increase in the incidence of bradycardia [19]. It is related to decreases in plasma catecholamine concentrations and the sympathetic outflow caused by α2-adrenergic activation. The incidence of bradycardia was higher in studies where the dexmedetomidine initial loading dose was infused over a short duration (5 min) [6]. However, bradycardia was transient and reversed with intravenous atropine. In our study, the loading dose was infused over a 10 min, and there were no statistically significant differences (1 vs 3 vs 3) among the three groups.
In this study, we investigated the effects of dexmedetomidine on spinal anesthesia with only two doses, 0.5 and 1.0 ug/kg. Therefore, it is difficult to discuss the dose-response relationship of the dexmedetomidine dose and the spinal anesthesia duration. Further studies are needed to determine the dose-response relationship.
In conclusion, both 0.5 and 1.0 ug/kg of dexmedetomidine administered as isolated boluses in the absence of maintenance infusions prolonged the duration of spinal anesthesia. In addition dexmedetomidine group showed the higher sedation score than in the control group without oxygen desaturation.