Prolonged post-reperfusion syndrome during multivisceral organ transplantation in a pediatric patient: a case report
Article information
Abstract
Multivisceral organ transplantation involves the transplantation of three or more abdominal organs, including small bowel, duodenum, stomach, liver, pancreas, colon, and so on. The large amounts of cold and acidic loading into systemic circulation from the graft during multivisceral organ transplantation may result in severe post-reperfusion syndrome (PRS). We describe here a 6-year-old pediatric patient with chronic intestinal pseudo-obstruction who experienced prolonged PRS and severe metabolic acidosis during seven abdominal organ transplantation including the liver, spleen, stomach, duodenum, small bowel, colon and pancreas. The hypotensive period lasted approximately 10 minutes after graft reperfusion and was accompanied by severe metabolic acidosis and hypothermia. Since PRS can be easily associated with adverse outcomes, such as poor early graft function and primary non-function, not only meticulous surveillance for aggravating factors for PRS but also their immediate correction were necessary in managing a pediatric patient undergoing multivisceral organ transplantation.
Multivisceral organ transplantation includes the transplantation of three or more abdominal organs, generally including the small bowel, duodenum, stomach, liver, pancreas, and colon [1]; in some patients with renal failure, the kidneys can also be included as an en-bloc graft [2].
Post-reperfusion syndrome (PRS) during liver transplantation is defined as ≥ 30% decrease in mean arterial pressure occurring within 5 minutes after reperfusion and lasting for at least 1 minute [3]. PRS can result in adverse outcomes, including poor early graft function, primary graft non-function, and death [3,4]. Thus, PRS may be able to be adopted as an index for determining outcome in liver transplantation. PRS may be caused by cold and acidic ingredients released during reperfusion and/or by the expression of proinflammatory cytokines during ischemia [5].
Severe hemodynamic instability and significant metabolic disturbances during graft reperfusion have been reported in patients undergoing small bowel transplantation only [6]. Less is known, however, about PRS during transplantations for multivisceral organs including liver and small intestine.
We encountered a pediatric patient who experienced prolonged hemodynamic instability with severe metabolic acidosis and hypothermia shortly after graft reperfusion during transplantation of seven abdominal organs and then performed retransplantation of liver due to hepatic failure.
Case Report
A 6-year-old girl 102 cm tall and weighing 17 kg, with chronic intestinal pseudo-obstruction was scheduled for multivisceral organ transplantation. Four-years earlier, she visited a hospital for abdominal distention and was diagnosed with gastric volvulus and congenital megacolon, for which she underwent gastropexy and segmental resection of the transverse colon. Since her symptoms did not improve over the next year, she was referred to our center and underwent a transverse colostomy. After that, she was repeatedly hospitalized and maintained on total parenteral nutrition due to continuously recurring mechanical ileus, malnutrition, and electrolyte imbalance. Two months before surgery, she was admitted due to abdominal distension and a malfunctioning colostomy. The colostomy function was normalized 2 days later, and she received conservative medical care, including total parenteral nutrition.
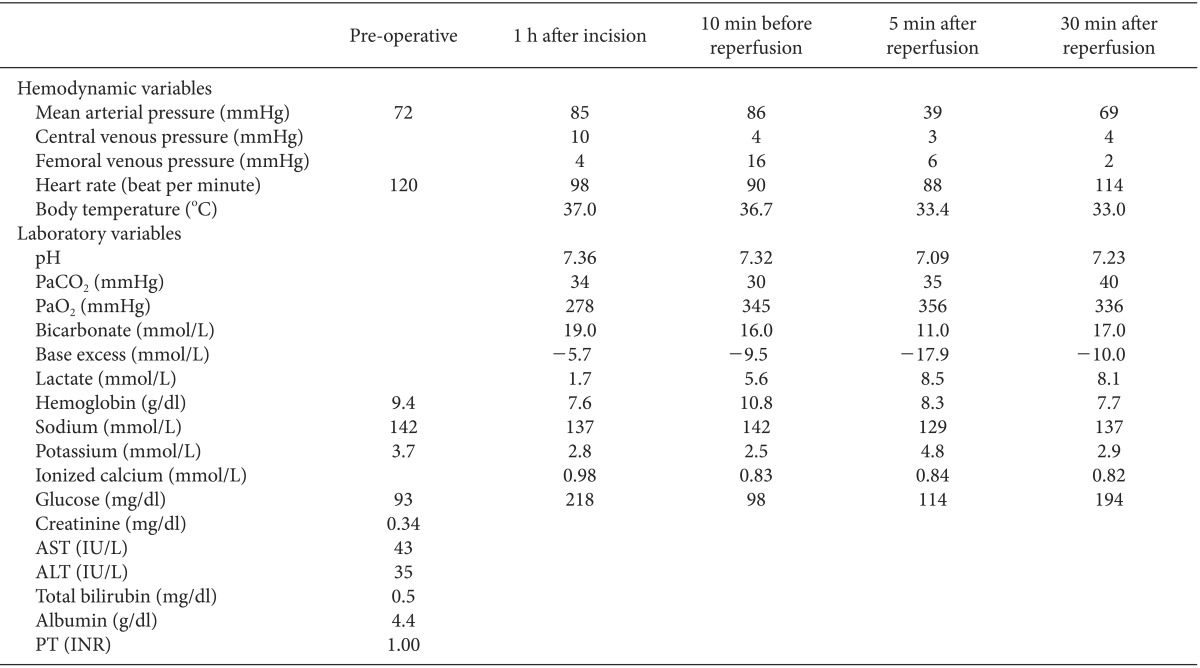
The patient was scheduled for multivisceral organ transplantation, including seven abdominal organs, the liver, spleen, stomach, duodenum, small bowel, colon and pancreas. Cadaveric donor was 5-year-old female 110 cm tall and weighed 21 kg, who had brain death due to increased intracranial pressure caused by medulloblastoma. Histidine-tryptophan-ketoglutarate solution was used as preservation solution and ischemic time was 170 min. Intraoperative gross finding of the grafts showed no significant abnormality. The recipient was not premedicated and arrived at the operating room with her father. Her blood pressure was 120/64 mmHg, her heart rate was 120 beats/min, and her oxygen saturation on pulse oxymetry was 100% before induction of anesthesia. Pre-operative blood laboratory data were within normal ranges, including hemoglobin (9.4 g/dl), Na+ (142 mmol/L), K+ (3.7 mmol/L), creatinine (0.34 mg/dl), aspartate transaminase (AST; 43 IU/L), alanine transaminase (ALT; 35 IU/L), total bilirubin (0.5 mg/dl), albumin (4.4 g/dl), and prothrombin time (1.00 INR). Anesthesia was induced with 75 mg of pentothal sodium, 15 mg of rocuronium, and 50 µg of fentanyl. After tracheal intubation, anesthesia was maintained with 1% sevoflurane in 50% oxygen in air and continuous infusion of fentanyl (100 µg/h) and vecuronium (2 mg/h). Her brachial artery was cannulated for continuous arterial blood pressure monitoring. A central venous catheter was inserted into her right internal jugular vein and a second into her subclavian vein to monitor central venous pressure and venous oxygen saturation. Femoral venous pressure was also monitored. Ten minutes before graft reperfusion, her vital signs and arterial gas analysis were as follows; mean arterial blood pressure (MAP) was 86 mmHg, heart rate was 90 bpm, central venous pressure was 4 mmHg, femoral venous pressure was 16 mmHg, and body temperature was 36.7℃, pH was 7.32 with a base excess of -9.5 mEq/L, arterial lactate concentration was 5.6 mmol/L, Na+ was 142 mmol/L, K+ was 2.5 mmol/L, Ca2+ was 0.83 mmol/L, hemoglobin was 10.8 g/dl, and blood glucose was 98 mg/dl.
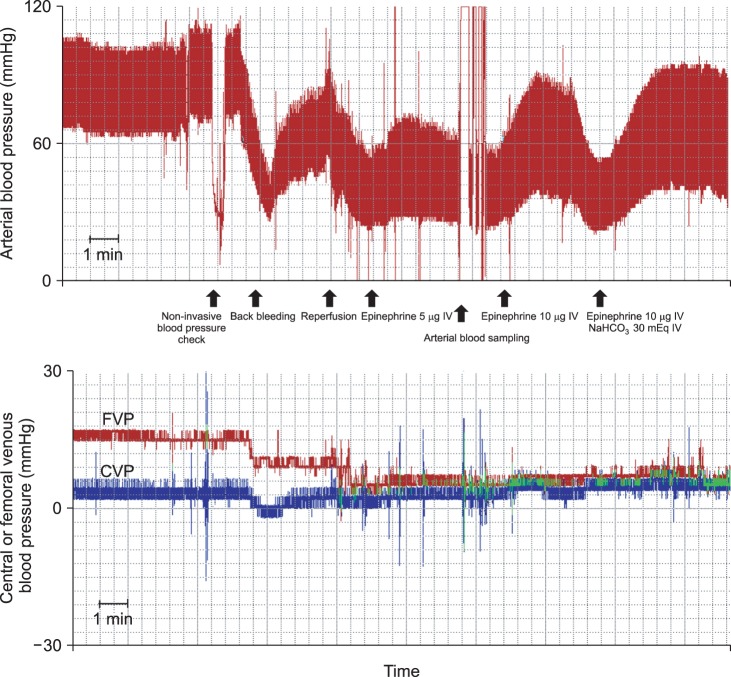
After injection of 20 mEq of NaHCO3, graft reperfusion was performed. Just after reperfusion, MAP rapidly decreased to 39 mmHg. Femoral venous pressure decreased after IVC unclamping from 16 mmHg to 4 mmHg and central venous pressure was maintained around 4 mmHg. Because the decrease of arterial pressure was too severe to expect spontaneous recovery and met the definition of PRS (≥ 30% decrease in MAP occurring within 5 minutes after reperfusion and lasting for at least 1 minute). She was immediately administered 5 µg of epinephrine, resulting in an MAP of 44 mmHg. However, repeated injections of 10 µg epinephrine and volume replacement were required to increase her MAP due to prolonged hypotension. Hypotension was sustained over 10 minutes, during which her averaged arterial pressure was 38 mmHg despite intermittent surge of MAP > 60 mmHg (Fig. 1). Arterial blood gas analysis 5 minutes after reperfusion showed severe metabolic acidosis, with a arterial blood pH of 7.09 with a base excess of -17.9 mEq/L, and the arterial lactate concentration was 8.5 mmol/L, K+ was 4.8 mmol/L, Ca2+ was 0.84 mmol/L, glucose was 114 mg/dl, and hemoglobin was 8.5 g/dl. To correct metabolic acidosis and hypotension, she was immediately and repeatedly injected with 30 mEq of NaHCO3 and 10 µg of epinephrine. Her body temperature had decreased to 33.4℃ just after reperfusion, with hypothermia sustained for 30 min (nadir body temperature: 33.0℃) despite active warming with an air warmer. Subsequently, continuous infusion of norepinephrine (0.1 µg/kg/min) until the end of the operation was required to maintain her MAP within an acceptable range. At the end of surgery, her body temperature increased to 35.7℃, but metabolic acidosis was sustained despite frequent administration of NaHCO3 (arterial pH: 7.23; base excess: -10.0 mEq/L). During the surgery, the patient was infused with a total of 2,900 ml of fluid, including 1,500 ml of balanced crystalloid solution, 100 ml of half-normal saline, 300 ml of 5% dextrose water, and 1,000 ml of 5% albumin, as well as 2 units of packed red blood cells. After 9 hours of surgery, the patient was transferred to intensive care unit. Hemodynamic and laboratory changes during the operation are shown in Table 1.
After surgery, the AST and ALT concentrations of the patient increased rapidly (immediate postoperative AST 2,108 IU/L and ALT 2,351 IU/L), reaching 7,300 IU/L and 6,400 IU/L, respectively, on the second day after the operation. She required continuous infusion of fresh frozen plasma due to prolongation of prothrombin time (3.07-5.69 INR). Her total bilirubin concentration, which was 0.5 mg/dl preoperatively, increased to 3.4 mg/dl postoperatively. The patient was diagnosed with primary hepatic graft failure and scheduled for re-transplantation of the liver. An adult to child living donor liver transplantation was performed on the third day after multivisceral transplantation. During this operation, the patient's vital signs remained stable until reperfusion, including a MAP of 70-80 mmHg. Ten minutes before reperfusion, her arterial blood pH was 7.49 with a base excess of -0.1 mEq/L, arterial lactate concentration was 3.8 mmol/L, Na+ was 137 mmol/L, K+ was 3.3 mmol/L, Ca2+ was 0.97 mmol/L, hemoglobin was 8.2 g/dl, and blood glucose was 170 mg/dl. After reperfusion, PRS developed again and lasted over 1 min. She received three injections each of 4 µg epinephrine and 20 µg of phenylephrine to maintain MAP within an acceptable level. Her arterial lactate concentration was 3.1 mmol/L at pH 7.44 with a base excess of -2.2 mEq/L, Na+ concentration was 138 mmol/L, K+ was 2.9 mmol/L, Ca2+ was 1.00 mmol/L, and hemoglobin was 6.8 g/dl. After transfusion of one unit of packed red blood cells, her vital signs remained stable (MAP: 70-76 mmHg) until the end of the operation. At that time, plasma hemoglobin concentration was 11.9 g/dl, AST was 453 IU/L, ALT was 695 IU/L, total bilirubin was 3.6 mg/dl, creatinine was 0.6 mg/dl, albumin was 2.9 g/dl, and prothrombin time was 1.74 INR. Her serum AST and ALT concentrations decreased gradually. On the seventh day after liver transplantation, AST and ALT concentrations had declined to 69 IU/L and 150 IU/L, respectively, her total bilirubin was 1.4 mg/dl, prothrombin time was 1.07 INR, and feeding through gastrostomy tube was started. The patient was managed conservatively for 3 months including 50 day stay in the Intensive Care Unit and discharged in an improved condition 139 days after multivisceral transplantation.
Discussion
We describe a pediatric patient who experienced severe PRS with metabolic acidosis and hypothermia during multivisceral organ transplantation. The hypotensive period lasted for about 10 minutes after the reperfusion. Following multivisceral transplantation, this patient experienced hepatic graft failure, requiring re-transplantation of the liver alone on the third postoperative day.
Since the first descriptions of small bowel and multivisceral abdominal transplantation in 1960 [7,8], this procedure has become the standard treatment for children with intestinal failure who experience difficulties with total parenteral nutrition [6,9]. Graft survival rates have increased yearly due to advanced immunosuppression protocols and surgical techniques [9], with 1-year and 5-year graft survival rates 77.7% and 55.0%, respectively [10]. Small bowel transplantation has been recommended for children who cannot receive at least half of their expected caloric requirements enterally and who also suffering from growth failure, deterioration of liver function, loss of venous access, and/or recurrent sepsis [11]. Diagnoses suggesting the need for small bowel transplantation include volvulus, intestinal atresia, gastroschisis, pseudo-obstruction, Hirschsprung's disease, and trauma [9]. Pretransplant work-up, including gastrointestinal contrast studies and liver biopsy, is required to determine whether to transplant small bowel alone or with other organs [9].
Graft reperfusion during small bowel transplantation may cause PRS, resulting in severe hemodynamic instability and metabolic disturbances [6]. Analysis of 27 patients who underwent small bowel transplantation showed decreases in MAP from 74.6 ± 5.0 mmHg during preenterectomy to 65.8 ± 8.2 mmHg 5 min after reperfusion (12% decreased); decreases in systemic vascular resistance index from 1,303 ± 228 dyne·m2/cm5 during preenterectomy and 980 ± 242 dyne·m2/cm5 5 min after reperfusion (25% decreased); and decreases in pH from 7.41 ± 0.06 during preenterectomy and 7.38 ± 0.05 5 min after reperfusion (0.4% decreased) [12]. Thus, ischemia-reperfusion injury to the small intestine may result in metabolic changes during the post-reperfusion period [13]. In our case, MAP of the patient decreased from 86 mmHg before reperfusion to 39 mmHg after reperfusion (55% decreased), whereas pH decreased from 7.32 to 7.09 (3% decreased). Thus, PRS in our case was more severe than previously reported cases, although systemic vascular resistance was not measured in our case.
Unlike in small bowel transplantation and liver transplantation, PRS has not been well-documented in multivisceral transplantation. Additionally, the definition of PRS after simultaneous reperfusion of multi-organ is unclear, thus prevention strategy of PRS is also not established in multivisceral transplantation. It is likely, however, that the degree of PRS would be similar or greater following multivisceral transplantation because en-bloc grafts contain multiple organs, including the small bowel and/or liver. The patient described here experienced prolonged hypotension, severe metabolic acidosis, hypothermia, and mild hypocalcemia. The period of PRS was sustained for over 10 minutes, until severe metabolic acidosis was improved and norepinephrine was continuously infused. However, mild metabolic acidosis still remained, even after repeat injections of NaHCO3, and hypothermia was not corrected for over 30 min after reperfusion, despite active warming.
Several hypotheses can be advanced to explain the prolonged PRS and severe metabolic acidosis in this patient. First, the large size of the en-bloc graft, which included several organs, may have caused large amounts of acidic loading and hypothermia. Our patient, who weighing only 17 kg, underwent en-bloc transplantation of the liver, spleen, stomach, duodenum, small bowel, colon and pancreas, suggesting that the graft volume was relatively high and may have caused severe acid loading resulting in severe PRS. Second, poor graft status may worsen the severity of the metabolic acidosis after graft reperfusion. Silberhumer et al. [14] defined initial poor graft function as AST > 2,500 UI/L and clotting factor support for over 2 days; and primary graft non-function as a requirement for re-transplantation within 7 days. In our patient, immediate postoperative AST and ALT was 2,108 IU/L and 2,351 IU/L, respectively, and then increased rapidly, exceeding 7,000 IU/L and 6,000 IU/L, respectively, on the second postoperative day. In addition, since prothrombin time extended over 3.0 INR, the patient required continuous transfusion with fresh frozen plasma. Although we cannot assure that the most important cause of primary non-functioning of hepatic graft after multivisceral transplantation in our case was due to the prolonged severe PRS or poor initial graft dysfunction from the cadaveric donor, it is possible to consider that poor initial hepatic graft dysfunction and subsequent severely prolonged PRS may further aggravate hepatic graft dysfunction, because the significant associations between low MAP and negative surgical outcomes, including initial poor graft function, primary graft non-function, or death has been reported [4,15].
As shown in the present case, severe PRS, including hemodynamic instability, hypothermia, and metabolic disturbance, were encountered at the reperfusion of organ grafts in a pediatric patient undergoing multivisceral organ transplantation. Meticulous surveillance and immediate management of aggravating factors for PRS, such as hypotension, acid-base imbalance, electrolyte imbalance, and hypothermia, may be indicated for managing patients undergoing multivisceral organ transplantation.