Acute onset Lance-Adams syndrome following brief exposure to severe hypoxia without cardiac arrest -a case report-
Article information
Abstract
Myoclonic status epilepticus (MSE) within the first 24 hours after cardiopulmonary resuscitation (CPR) predicts poor prognosis, enough to discontinue the treatment. In contrast, chronic MSE appearing a few days after CPR is called Lance-Adams syndrome (LAS), which is characterized by preserved intellect and a favorable prognosis. We herein report a case of LAS, which developed after a transient hypoxic event without an overt cardiac arrest due to hematoma formation in the neck after partial glossectomy. Differential diagnosis was also challenging as LAS was developed 8 hours after the hypoxic event.
Brief episode of severe hypoxemia is not uncommon in the field of anesthesia. It can be caused by various situations, such as difficult airway management, airway obstruction, drug-induced respiratory depression, malfunction of anesthetic machine, pulmonary embolism, and hypotension. However, most cases rarely result in fatal outcomes since professional equipment and well-trained anesthesiologists are almost always in the operating room.
We present a patient who underwent partial glossectomy and suffered severe hypoxemia without cardiac arrest due to neck hematoma in the recovery room. Hypoxemia was quickly treated with decompression and bag-mask ventilation. However, myoclonus was observed 8 hours after the event, and shortly afterwards, he was diagnosed with Lance-Adams syndrome (LAS). With this case, we review LAS that is known as a rare neurological complication seen in survivors of cardiac arrest [1]. Also, we discuss predictive factors for poor neurological outcome even after transient hypoxemia without cardiac arrest, and highlight the importance of providing them with intensive care.
Case Report
A 48-year-old man underwent right partial glossectomy with neck dissection for tongue cancer under general anesthesia. Past medical history showed that he had been diagnosed with diabetes mellitus (DM) for five years and that he is a 30 pack-year smoker. During the five hours of operation, his vital signs were kept stable and blood sugar levels were well controlled between 82-84 mg/dl. Pulse oxygen saturation (SpO2), pulse rate, and non-invasive blood pressure (NIBP) were stable during immediate postoperative period in the recovery room.
He began complaining of troubled breathing and showed agitation 30 minutes after arrival in the recovery room. We informed his otolaryngologist of the situation and relieved the dressing bandage around his neck. As his oxygen saturation gradually decreased to 0%, we started bag-mask ventilation. NIBP was checked, 149/92 mmHg. SpO2 was immediately recovered to 96%. Although severe hypoxemia occurred, it did not lead to cardiac arrest, which was known from palpable pulse of radial artery throughout the course. His heart rate remained above 40 beats/min. At the same time, his otolaryngologist arrived and immediately opened the incision site for decompression. At once, he was able to breathe and his saturation was maintained 100%.
He was transported to the operating room to remove the hematoma of his neck. Intubation was performed without difficulty and mechanical ventilation was applied with FiO2 50%. All vital signs were unremarkable (SpO2 98-99%, heart rate 80-120 beats/min, and temperature 36.0-36.2℃), except blood pressure. It was checked below 90/60 mmHg twice during the 2 hours of operation, and ephedrine 10 mg was injected. On average, the mean arterial pressure was kept between 60-80 mmHg. At the end of the operation, his condition was stable and appropriate for extubation. Bispectral index (BIS) was increased to 90 and his eyes were opened spontaneously; he was extubated. However, soon after extubation, he showed generalized tonic-clonic seizure and it was only discontinued by inhalation of sevoflurane and administration of thiopental. He was reintubated and phenytoin, lorazepam, and midazolam were administered to control the seizures. He was admitted to the intensive care unit (ICU) in a sedated state. Light reflexes were normal. Blood glucose level was 200 mg/dl.
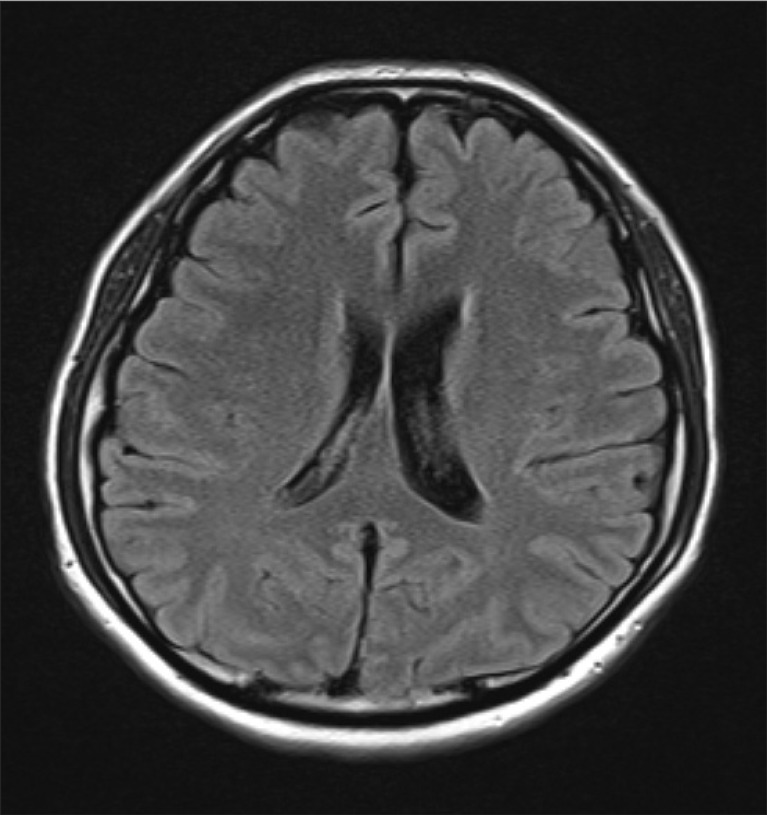
Six hours later, when a tactile stimulus was applied, he developed widespread myoclonic seizures on all four limbs, even under infusion of large dose of anticonvulsants. Two days later, he was still deeply sedated to control myoclonic seizures, but the frequency and duration of seizures were only mildly reduced. His brain computed tomogram (CT) showed no remarkable evidence of hypoxic brain damage and his electroencephalogram (EEG) study showed normal findings. On day 10 of hospitalization, his sedatives were completely withdrawn and he became alert with a frowned face and myoclonus affecting both knees and ankles. The treatment with sodium valproate and levetiracetam were continued for recovery. Three weeks later, magnetic resonance (MR) brain imaging did not show any abnormalities (Fig. 1). Two months after surgery, he showed ataxic movement and gait disturbance. His myoclonic action has been well controlled by medications. He has been on intensive rehab for his recovery.
Discussion
Myoclonic status epilepticus (MSE) within the first 24 hours after cardiopulmonary resuscitation (CPR) is generally considered as an accurate predicting factor of deleterious outcome enough to discontinue the treatment [2]. It is limited to '24 hours' because MSE is categorized into acute and chronic types, which have completely different prognosis [3]. Chronic MSE is called Lance-Adams syndrome, mostly shown a few days after CPR. Since LAS shows much favorable prognosis compared to that of acute MSE, it is important to remember the different clinical features of LAS. Moreover, LAS can develop acutely, which can lead to misdiagnosis even more easily, as in our case. In addition to the time course, LAS is distinguished from acute MSE by several clinical features, such as awareness, intention myoclonus, and normal brain CT or EEG. The pathophysiology of LAS is elusive, but the mechanism of hypoxic brain injury has been assumed to be an important role. It is based on the fact that patients in previous reports of LAS were the survivors of 'hypoxic' cardiac arrest rather than 'circulatory' cardiac arrest [3-5]. Patients with pure hypoxic event seem to have far better outcome than those with circulatory cardiac arrest. There are several presumed mechanisms for this [6]. Hypoxia produces hypercapnia, which results in increase of cerebral blood flow secondary to cerebrovascular dilation. This results in the brain to be continuously supplied with nutrients and glucose and toxic metabolites to be removed by the circulation. Toxic metabolites, including lactic acid [7] and free radicals [8] damage not only astrocytes, but glial and mesenchymal cells as well. In contrast, pure hypoxia leads to only mild astrocytic swelling without brain necrosis [9].
Time course in the present case showed acute MSE that have easily led us to misdiagnosis. It is important to differentiate between the two types of MSE because therapeutic strategy must be established accordingly. However, misdiagnosis can occur. Patients with LAS are aware, but it can be masked by deep sedation to control seizure. EEG and brain CT can be delayed since MSE is difficult to control even with high dose of anticonvulsants. Moreover, as seen in our case, some patients with LAS can start myoclonic activity within 24 hours after CPR [10]. Not misdiagnosing LAS, we should well acquaint ourselves with other predictors of poor outcome after CPR comprehensively; absent pupillary light response or corneal reflexes at 72 hours, extensor or no motor response to pain at 72 hours, bilateral absent cortical responses on somatosensory evoked potentials at 72 hours, and serum neuron-specific enolase higher than 33 µg/L.
The present case might be regarded as a case of good outcome in spite of post-anoxic MSE. Ironically, we did not expect any neurological complication at the time of the event in the recovery room, as anoxic time was around 3 minutes and cardiac arrest had not occurred. Berek et al. [11] have shown that the most important determinant of hypoxic injury was the duration of anoxia, which is defined as the interval between collapse and the beginning of CPR. Patients with favorable outcomes were anoxic for 4.1 minutes, compared to 8 minutes for patients with unfavorable outcomes. Only one report described the occurrence of LAS without cardiac arrest, which was assumed to be caused by fat embolism [10]. The survivor was a young healthy man involved in a traffic accident and his SpO2 was decreased to 89% briefly without hypotension during orthopedic surgery.
It is worthy of notice what caused neurologic complications after brief hypoxemia without cardiac arrest. We can presume that the following factors may have contributed to the development of LAS; patient's vulnerability, co-morbid conditions, surgical factor, and lack of post-hypoxic management. The patient was a heavy smoker who had a history of DM and tongue cancer. Hyperglycemia is widely known as the main cause of endothelial dysfunction and neuronal cell death [12]. Although his blood glucose had been well controlled, diabetic patients show more exaggerated cortical vasoreactivity and more regional cortical atrophy than non-diabetic patients [13]. Long-lasting cigarette smoking is related to the increase in the level of inflammatory state and endothelial injury, which lead to impair vascular function and blood brain barrier integrity [14]. In addition, the hematoma in the neck may have compressed the jugular vein and/or the carotid artery compromising the cerebral blood flow as well, although circulatory arrest was not evident. These factors could make our patient vulnerable to hypoxemia inhibiting cerebrovascular dilation and a maintenance of cerebral blood flow, which made similar processes to circulatory cardiac arrest. Thus, if patients who are predisposed to hypoxic injury were exposed to brief severe hypoxemia without cardiac arrest, they should be regarded as survivors of cardiac arrest and be provided with appropriate post-resuscitation managements [15]. In this case, blood pressure was not elevated enough to ensure adequate cerebral blood flow during 2 hours of surgical removal of hematoma after recovery from hypoxemia. Blood glucose level was also elevated to 200 mg/dl. The mean arterial blood pressure is recommended to be maintained between 80-100 mmHg, at least for the first 24 hours after cardiac arrest. Blood glucose levels between 70-140 mg/dl are known to be associated with good neurologic outcome. Since hyperthermia worsens neurologic outcome, fever must be avoided.
It is clear that acute onset MSE does not always mean a poor neurologic outcome, but it does need more cautious approaches of diagnosis for several reasons. Short episodes of hypoxemia can occur at any time of anesthesia and bring detrimental neurologic consequences. Therefore, if those people with high risk of hypoxic injury have experienced hypoxemia, even for a short time, we should closely monitor them. Although it is not proven clearly, we may consider providing them an intensive post-resuscitation management to minimize unexpected complications.
Acknowledgments
This work was supported by clinical research grant from Pusan National University Hospital 2012.