Usefulness of a Cook® airway exchange catheter in laryngeal mask airway-guided fiberoptic intubation in a neonate with Pierre Robin syndrome -A case report-
Article information
Abstract
The case of a 33-day-old boy with Pierre Robin syndrome using a Cook® airway exchange catheter in laryngeal mask airway-guided fiberoptic intubation is presented. After induction with sevoflurane, classical reusable laryngeal mask airway (LMA) #1 was inserted and ultrathin fiberoptic bronchoscope (FOB) was passed through. A Cook® airway exchange catheter (1.6 mm ID, 2.7 mm OD) was passed through the LMA under the guidance of the FOB but failed to enter the trachea despite many trials. Then, an endotracheal tube (3.0 mm ID) was mounted on the FOB and railroaded over the FOB. After successful intubation, the Cook® airway exchange catheter was placed in the midtrachea through the lumen of the endotracheal tube. Even though the tracheal tube was accidentally displaced out of the trachea during LMA removal, the endotracheal tube could be easily railroaded over the airway exchange catheter.
Pierre Robin syndrome is a congenital malformation of craniofacial development that is described as a triad of micrognathia, cleft palate, and glossoptosis. Infants with this syndrome can create a challenge for the anesthesiologist during the induction of anesthesia and when managing the airway because of an underdeveloped or repositioned mandible that places the tongue into a posterior and elevated position in oropharynx, resulting in severe airway obstruction. After induction, visualization of the larynx under a direct laryngoscope is difficult or almost impossible in patients with Pierre Robin syndrome. Therefore, various techniques such as a paraglossal approach, using a straight Miller blade combined with a gum-elastic bougie [1], fiberoptic bronchoscopy [2,3], laryngeal mask airway-guided fiberoptic intubation [4,5], and intubation with a lighted stylet [6] have been reported as successful intubations in these cases. In a recent survey, most anesthesiologists reported tending to use LMA as a conduit for the bronchoscope, which shows that this technique is now firmly established in an approach to the difficult pediatric airway [7].
We report a successful case of orotracheal intubation using the Cook® airway exchange catheter (CAEC) to prepare for any accidental removal of the tracheal tube during the removal of the LMA following the laryngeal mask airway-guided fiberoptic intubation.
Case Report
A 3.09 kg, 33-day-old boy with Pierre Robin syndrome was admitted to our hospital for a glossopexy. The patient was born at 39 weeks by C-section and was diagnosed with the syndrome during pregnancy. After birth, the patient showed a micrognathia, retrognathia, and a large tongue. The patient also showed moderate chest retraction with coarse breathing sounds presenting difficulty in maintaining patent upper airway. He was kept in a prone/lateral position under 5 L/min of O2 hood at NICU until the operation.
In the operating room, he showed moderate chest retraction after changing the position from prone to supine. Intraoperative monitoring devices such as an ECG, an automatic blood pressure cuff, and a pulse oximeter were applied to the patient. The initial oxygen saturation was 98% in room air and other vital signs (BP: 96/27 mmHg, PR: 161/min, RR: 42/min) were in normal ranges. A mask was applied before induction and the manual ventilation was well maintained with patient's spontaneous ventilation. Premedication with 0.004 mg/kg of glycopyrrolate was given intravenously and anesthesia was induced with gradual increment of sevoflurane concentrations from 2% to 4% with oxygen by mask while also maintaining spontaneous respiration. Brief direct laryngoscopic examination with a curved McIntosh blade (#1) was performed and only the hypopharynx was seen. Classical reusable laryngeal mask airway (LMA) #1, in which two vertically placed bars were removed, was inserted and effective ventilation was assured by chest expansion, auscultation, and EtCO2 monitoring. While pausing the ventilation, ultrathin fiberoptic bronchoscope (FOB) (Olympus ENF type XP, 1.8 mm OD, length: 530 mm, Tokyo, Japan) was passed through the lumen of the LMA and the vocal cords were well visualized. Under the visualization of the vocal cords by the FOB, we passed the Cook® airway exchange catheter (CAEC) (1.6 mm ID, 2.7 mm OD) (Cook Medical Incorporated, Bloomington, IN, USA) through the LMA into the vocal cords. However, passing the CAEC into the vocal cords under the guidance of the FOB repeatedly failed because the CAEC frequently flexed backwards toward the esophagus. At that time, we recognized that the length of an uncuffed ID 3.0 mm endotracheal tube (length: 16 cm) was longer than that of LMA#1 (length: 11 cm). Therefore, through the LMA, the tracheal tube was mounted on the FOB and introduced into the trachea. Successful ventilation was verified by manual ventilation. To prepare for any accidental removal of the tracheal tube while removing the LMA, we inserted the CAEC into the lumen of the tracheal tube. After placing the CAEC in the midtrachea, LMA was carefully removed while tightly holding both the CAEC and tracheal tube simultaneously. Despite this, the endotracheal tube was displaced out of the vocal cords while removing the LMA. By holding the CAEC firmly inside the trachea, the displaced endotracheal tube was easily railroaded over the CAEC under the direct laryngoscopy. After intubation, endotracheal tube position was verified again by auscultation of equal bilateral breathing sounds and EtCO2. During the procedure, anesthesia was maintained with intermittent sevoflurane administration in oxygen (O2: 1 L/min, air: 1 L/min) through the LMA to prevent awakening and desaturation of the patient. The maximum time allowed for the procedure was until the SpO2 decreased lower than 95%. Atracurium 1.5 mg was given intravenously for muscle relaxation and mechanical ventilation was maintained during the operation. The glossopexy was performed and the surgery was successfully completed. The extubation was carried out when the patient was in a completely alert state and the spontaneous ventilation was well maintained without any chest retraction following the extubation. The patient was then transferred to NICU in the supine position under 5 L/min of O2 mask.
Discussion
Patients with Pierre Robin syndrome may create difficulties during intubation and airway control is a major concern for anesthesiologists. Ventilation using a face-mask in the presence of a markedly recessed mandible may be difficult and patient's supine position frequently leads to a total obstruction of the airway. Visualization of the larynx is almost impossible once the patient is anesthetized. In a recent survey conducted amongst pediatric anesthetists in Canada, 73% stated that they first attempted a direct laryngoscopy and, in the case of a failure, 51% would choose laryngeal mask airway-guided fiberoptic intubation [7]. When FOB is chosen to be used for intubation, most anesthesiologists generally tend to use LMA as a conduit for the bronchoscope, indicating that this technique is now firmly established in an approach to the difficult pediatric airway.
Although this approach seems to be very successful, one problem is that the endotracheal tube can be easily withdrawn from the trachea during the removal of the LMA. In order to prevent this, Selim et al. [8] elongated the tracheal tube by connecting it to another smaller sized tube. Therefore, it was possible to keep the intubated tube patent inside the trachea while LMA was being removed, but instability of an elongated tube has still been a problem [8]. In addition to this, some authors [2,3] used a long guide wire inserted into the suction port of the fiberscope, which was then placed inside the trachea under direct vision. Having the guidewire left in the trachea, the fiberscope and LMA were withdrawn, and the tracheal tube was railroaded over the guidewire. A disadvantage of this method was that the guidewire can be easily bent and flipped out of the trachea. Osses et al. [9] introduced a method of using an adult intubating stylet attached to the end of the tracheal tube so that LMA would be removed while pushing the stylet inward.
As an alternative approach, we used the CAEC under the guidance of the fiberscope to ease the insertion of the endotracheal tube into the trachea. However, when this catheter was passed down through the LMA, it frequently emerged from the posterior aspect of the mask aperture of the LMA and resulted in an esophageal intubation even though vocal cords were well-visualized with the ultrathin FOB. Despite many trials using a CAEC, it was very difficult to approach the vocal cords with the tip of the catheter. Brimacombe and Berry [10] have reported that the success rate of blind placement of the CAEC via the LMA in adults was only 30% despite good LMA positioning. Therefore, this indicates that, even when the LMA is perfectly positioned, blind tracheal intubation cannot always be performed successfully. In children, perfect positioning of the LMA was observed in 44% [11], 29% [12], and 49% [13] of all cases, which also indicates that there may have been some impediment to blind passage of the endotracheal tube, a bougie or introducer when this technique is attempted.
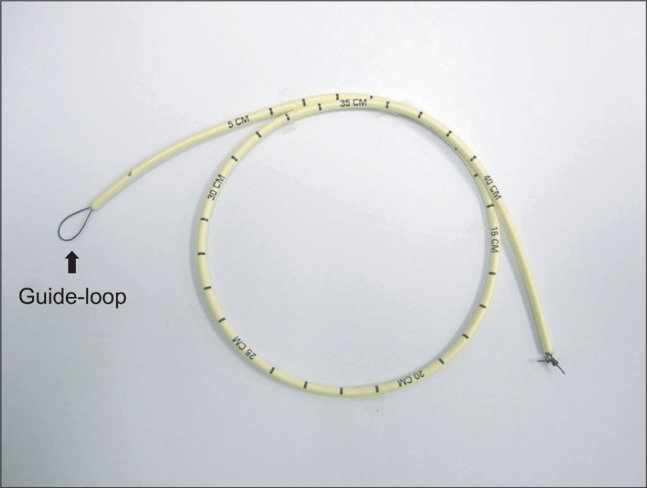
To correctly place the CAEC into the trachea, the guidewire technique was used. After the guidewire passed through the suction channel of the FOB, a stiffening device such as the CAEC or ureteral dilator railroaded over the guide wire through the LMA might be helpful in placing the CAEC or dilator correctly in the trachea [14]. In addition to this, we suggest another method for successful placement of the CAEC. As shown in Fig. 1, a fishing line is passed through the lumen of a CAEC and form a guide-loop like the one of Arndt endobronchial blocker (Cook® Medical Incorporated, Bloominton, IN, USA) at the distal end of the CAEC and a tight knot is made at the proximal end of the catheter, making sure that it does not come loose. The FOB is passed through the guide-loop (Fig. 1) and place the CAEC is placed in the midtrachea. Then, the FOB and LMA were removed while keeping the catheter in midtrachea and the endotracheal tube is mounted on the CAEC, followed by railroading over the CAEC. In a pediatric patient, weighing 7.5 kg, Thomas and Parry [15] also used a CAEC through which a FOB was inserted and the tip of the FOB was extended from the distal aperture of the CAEC. Following introduction of CAEC with the enclosed fiberscope into the trachea, the FOB was removed leaving the CAEC within the trachea. Although this is an interesting approach, considering that they used an Olympus LF-P fiberscope (proximal OD 2.2 mm and distal OD 1.8 mm) and a CAEC (2.3 mm ID for use with tracheal tubes of ID 4 mm or larger), it cannot be used in a smaller pediatric patient because the Olympus ENF type XP or LF-P fiberscope cannot pass through the lumen of the CAEC (1.6 mm ID for use with tracheal tubes of 3 mm or larger) which we used. Another problem with this approach is that the CAEC has to be cut to an adequate length to ensure that the FOB tip extends from the distal aperture of the CAEC. In our case, an uncuffed 3.0 mm ID endotracheal tube (length: 16 cm) was longer than that of LMA#1 (length: 11 cm). However, in pediatric difficult airway cases in which the endotracheal tube length is similar to or shorter than the LMA, the proximal end of the tracheal tube tends to disappear into the LMA once the tracheal tube has passed through the vocal cords. In this situation, it might be helpful to insert a CAEC first through the LMA using the suggested methods by Walker [14], Thomas and Parry's [15] methods, or our method in order to facilitate easy insertion of the CAEC into the trachea.
In summary, there may be advantages to use the CAEC in pediatric patients. First, it can provide a more stable passage for the endotracheal tube since the diameter of the CAEC which we used (1.6 mm ID) is larger than the guide wire previously used to pass through the suction port [2,3]. Second, it is much safer to attempt repeated intubation in the case of accidental tube displacement out of the trachea during removal of the LMA.