Current therapeutic agents and anesthetic considerations for diabetes mellitus
Article information
Abstract
As the incidence of diabetes mellitus (DM) continues to increase worldwide, more diabetic patients will be presented for surgery and anesthesia. This increase of DM is a consequence of the rise in new patients of type 2 DM, and is likely attributable to rapid economic development, improved living standards, aging population, obesity, and lack of exercise. The primary goal of management in DM is to delay, or prevent the macro- and microvascular complications by achieving good glycemic control. More understanding of the pathophysiology of DM has contributed to the advance of new pharmacological approaches. In addition to the conventional therapy for DM, glucagon-like peptide-1 (GLP-1) mimetics, dipeptidyl peptidase-4 (DPP-4) inhibitors, thiazolidinediones (TZDs), and insulin analogues are currently available effective hypoglycemic agents for the management of the patients with DM in the perioperative period and also consider the adverse effects of newly introduced agents that need more clinical observations.
Introduction
The International Diabetes Federation (IDF) reported in 2008, that 246 million adults worldwide had diabetes mellitus and prevalence was expected to reach 380 million by 2025 [1]. Korea National Health and Nutrition Examination Surveys (KNHANES) reported 9% of the adult population as the prevalence of DM in Korea in 2008 and Task Force Team for Basic Statistical Study of Korean Diabetes mellitus reported in 2007, that the number of patients with type 2 diabetes was expected to increase dramatically from about 3.5 million in 2010 (7.08% of the total population) to about 5.5 million (10.85%) by 2030 [2]. This increase of DM is a consequence of the rise in new patients of type 2 DM, and is likely attributable to rapid economic development, improved living standards, aging population, obesity, lack of exercise and Westernized lifestyle [3]. Type 2 diabetes is remained as the leading cause of cardiovascular disorders, blindness, end-stage renal failure, amputations, and hospitalizations. It is also associated with increased risk of cancer, serious psychiatric illness, cognitive decline, chronic liver disease, accelerated arthritis, and other disabling or deadly conditions. The pathophysiology of type 2 DM is characterized by peripheral insulin resistance, impaired regulation of hepatic glucose production and decreased beta cell function, eventually leading to beta cell failure. The primary goal of management in DM is to delay the macro- and microvascular complications by achieving good glycemic control and the relationship between tight glycemic control and microvascular disease in type 2 DM is established [4].
Based on the encountering more DM surgical patients needing anesthesia, anesthesiologists will be more involved in the perioperative care, as the number of these patients. The enhanced understanding of rapidly evolving medical treatment for DM is helpful for anesthesiologist to manage the diabetic patients in the perioperative period with a rational basis. This article will review the current literature and incorporate new concepts, agents for the care of the patients with DM.
Classification of Diabetes Mellitus
Type 1 diabetes is called insulin dependent diabetes mellitus (IDDM) which is caused by pancreatic beta cell destruction, which results from an autoimmune attack by the body itself, and is rendered incapable of making insulin. Type 2 diabetes results from a combination of insulin resistance and a relative deficiency of insulin that is usually associated with defective insulin secretion [5] (Table 1).
Diagnosis of Diabetes Mellitus
In 1997, an International Expert Committee on the Diagnosis and Classification of Diabetes Mellitus published a new classification scheme and revised diagnostic criteria for DM, from the 1979 National Diabetes data Group and 1985 WHO study group (the following criteria are from the 2012 revision) [6]. The Expert Committee recognized an intermediate group of individuals whose glucose levels do not meet the criteria for diabetes, however, still higher than those considered normal. Several criteria may be used, independently, to establish the diagnosis. Any finding falling within the positive criteria should be repeated on a subsequent day with another test in any criteria set: e.g., random plasma glucose with symptoms, might be followed-up with a fasting plasma glucose level.
Fasting plasma glucose (FPG) ≥ 126 mg/dl (7.0 mmol/L) on more than one occasion
-
Symptoms (such as polyuria, polydipsia, unexplained weight loss)
AND a random plasma glucose ≥ 200 mg/dl (11.1 mmol/L)
A 75 g oral glucose tolerance test with a 2 hour value of plasma glucose ≥ 200 mg/dl (11.1 mmol/L)
Hemoglobin A1c ≥ 6.5%.
The 1997 guidelines included recommending the use of fasting plasma glucose as part of the diagnostic tests, but the cut-off point was reduced from 140 to 126 mg/dl. Fasting plasma glucose values are preferred for their convenience, reproducibility, and correlation with increased risk of microvascular complication [7]. Since 1997, many studies relating to the diagnosis of DM have been reported and a number of questions have been raised about the use of fasting plasma glucose vs. oral glucose tolerance test. It has been known that impaired glucose tolerance is related with cardiovascular risk factors and events, whereas, impaired fasting glucose is less strongly related with cardiovascular events or mortality [8,9]. HbA1c has many advantages including an indication of glucose concentrations over a number of weeks and useful monitor of both diagnosis and response to treatment. Recently, the use of HbA1c, both as a diagnostic tool and as a predictor of perioperative outcomes has been studied and reported that HbA1c may have an important role in predicting perioperative outcomes in patients with DM undergoing a variety of surgical procedures [10,11].
Currently Used Drug Therapy
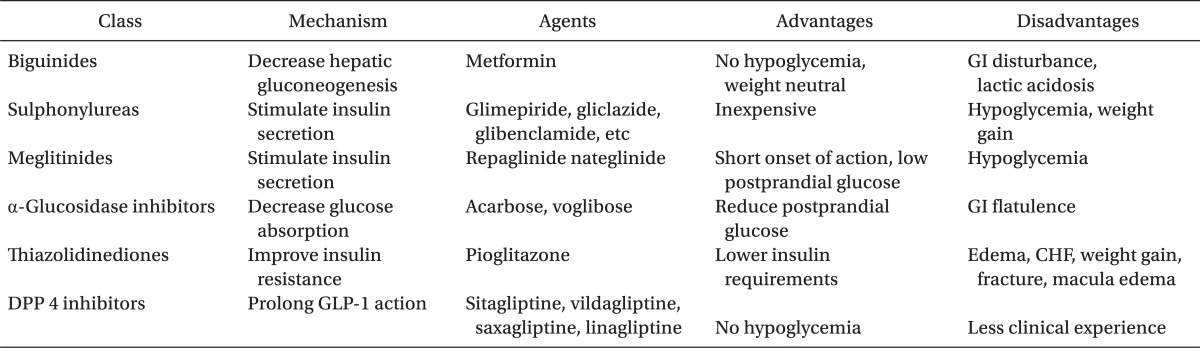
Diet and exercise are first line treatments along with hypoglycemic drugs to achieve the goal of improving glycemic control, as well as preventing both microvascular and macrovascular complications. There are currently used distinct classes of hypoglycemic agents: biguanides, sulfonylureas, meglitinides, thiazolidinediones, a-glucosidase inhibitors, incretin mimetics, DPP-4 (Dipeptidyl Peptidase-IV) inhibitors and insulin (Tables 2 and 3).
Biguanides
Biguinides are old agents that work by reducing hepatic glucose output and to a lesser extent, enhancing insulin sensitivity in hepatic and peripheral tissues [12]. Metformin still remains as the most widely used first-line for type 2 DM. Metformin should be prescribed to all people with type 2 DM, unless contraindicated. Current recommendations of the American Diabetes Association and European Association for the Study of Diabetes include metformin, diet and exercise as first-line therapy for the treatment of patients with type 2 DM, irrespective of the presence of overweight status [13]. It is generally considered weight-neutral with chronic use and does not increase the risk of hypoglycemia when used as monotherapy [14]. But, hypoglycemia can occur when used in combination with sulphonylureas or insulin. Metformin is related with initial gastrointestinal side effects, and caution is advised to avoid its use in patients at risk for lactic acidosis. Less than 60 ml/min of a glomerular filtration rate value would be the approximate equivalent of the above serum creatinine cutoffs (1.4 mg/dl in women and 1.5 mg/dl in men), and metformin should be discontinued [14]. It is very relevant to remember that patients who are about to receive intravenous iodinated contrast material with potential for contrast induced renal failure or undergo a surgical procedure with potential compromise of the circulation should have metformin stopped, until stable renal function can be established (normal urine output, normal serum creatinine and no evidence of fluid overload or circulatory compromise).
Sulfonylureas
Sulfonylureas were the mainstay of antidiabetic therapy since the early 1950s. The oldest oral agent class is the sulfonylurea insulin secretagogues. They work by stimulating insulin release from the insulin secreting β cells, which are located in the pancreas [15], and may slightly improve insulin resistance in peripheral target tissues (muscle, fat). First-generation sulfonylureas, such as chlorpropamide, tolazamide and tolbutamide, have a longer half-life, a greater incidence of hypoglycemia, more frequent drug interactions, and are now rarely used. A newer sulfonylurea, glimepiride, has a more rapid onset of action and a better coverage of the postprandial glucose rise and less risk of hypoglycemia [16]. They cause greater suppression of overnight hepatic glucose output, thereby, further lowering fasting blood glucose concentrations. These agents, especially the longer acting ones, have the potential to cause hypoglycemia, especially in elderly individuals. Hypoglycemia is usually related to delayed meals, increased physical activity, alcohol intake, or renal insufficiency. All sulfonylurea have been associated with weight gain, unless the diabetic diet and exercise program are followed [16]. The choice of sulfonylurea is primarily dependent upon the cost and availability, because their efficacy against microvascular and cardiovascular complications is similar [17].
Meglitinides
The meglitinides (Repaglinide and nateglinide) are structurally different than sulfonylureas, but their mechanism of action closely resembles that of sulfonylureas because they stimulate the release of insulin from the pancreatic beta cells through a different binding site on the sulfonylurea receptor. The meglitinides can be used as monotherapy, or in combination with other oral hypoglycemic drugs, like metformin, which results in superior glycemic control than with either agent used as monotherapy. Their clinical efficacy is similar to that of the sulfonylureas. Some potential advantages of this class of agents include a greater decrease in postprandial glucose and a decreased risk of hypoglycemia [18].
Thiazolidinediones
Thiazolidinediones (TZDs) improve insulin sensitivity in the skeletal muscle by increasing the efficacy of glucose transporters, lowering HbA1c and reducing both fasting and postprandial glucose concentrations [19]. They do not increase the risk of hypoglycemia when used as a single agents, and may be more durable in their effectiveness than sulfonylureas and metformin [20]. Pioglitazone appeared to have a modest benefit on cardiovascular events as a secondary outcome in one large trial involving patients with overt macrovascular disease [21]. Thiazolidinediones are associated with weight gain (2-3 kg), a small reduction in the hematocrit, and a mild increase in plasma volume. Peripheral edema and congestive heart failure are more common in individuals treated with these agents with the incidence ranging from 2.5% to 16.2%. This risk is increased with age, drug dose, female, impaired renal function, and concomitant insulin use. These agents are contraindicated in patients with liver disease or congestive heart failure (New York Heart Association class III or IV) [22].
α-Glucosidase inhibitors
α-Glucosidase inhibitors include acarbose and voglibose. They block the enzyme α-glucosidase, found in brush border cells of small intestine, cleaving more complex carbohydrates into sugars. These agents delay absorption of glucose and decrease meal-related blood glucose increases. Thereby, these agents are most useful in patients who have mild fasting plasma glucose elevations or in patients with predominant postprandial hyperglycemia [23]. The major side effects such as diarrhea, flatulence, and abdominal distension, are related to increased delivery of oligosaccharides to the large bowel, and can be reduced somewhat by gradual upward dose titration. These agents should not be used in individuals with inflammatory bowel disease, gastroparesis, or a serum creatinine > 2.0 mg/dl) [24].
Incretins/GLP-1(Glucagon-Like Peptide-1) agonists
Incretins (glucagon like peptide-1 and glucose dependent insulinotropic polypeptide) are enteroendocrine hormones released into the bloodstream from L cells of ileum and colon and K cells of duodenum and jejunum [25]. The effect of incretins refers to the augmented release of insulin from oral ingestion of glucose. The injectable GLP-1 receptor agonists mimic the effects of endogenous GLP-1, thereby, stimulating pancreatic insulin secretion in a glucose-dependent fashion, suppressing pancreatic glucagon output, slowing gastric emptying, and decreasing appetite (Table 3). Exenatide is an analogue of GLP-1. Exenatide has prolonged GLP-1-like action, and binds to GLP-1 receptors found in islets, the gastrointestinal tract, and the brain [26]. Liraglutide, another GLP-1 receptor agonist, is almost identical to native GLP-1 with a long half-life. GLP-1 receptor agonists increase glucose-stimulated insulin secretion, suppress glucagon, and slow gastric emptying [27]. Their main advantage is weight loss, which is modest in most patients but can be significant in some. A limiting side effect is nausea and less commonly, vomiting or diarrhea, particularly early in the course of the treatment. Incretin therapy appears to provide an effective alternative to the currently available hypoglycemic agents [28].
DPP-4 inhibitors
DPP-IV inhibitors inhibit degradation of native GLP-1 and thus, enhance the incretin effect. DPP-IV, which is widely expressed on the cell surface of endothelial cells and some lymphocytes, degrades a wide range of peptides (not GLP-1 specific). These enhance circulating concentrations of active GLP-1 [29]. DPP-4 inhibitors mimic the therapeutic effects of incretin mimetics including stimulation of insulin secretion, inhibition of glucagon secretion, possibly preservation of β-cell mass and inhibition of apoptosis. These drugs display similar efficacy in lowering HbA1c compared with other antihyperglycemic agents. They do not reduce the appetite or cause weight loss, such as GLP-1 agonists, and have a low potential for hypoglycaemia when used as monotherapy [30]. One safety concern involves the potential of DPP-4 inhibitors to interfere with the immune functions: a meta-analysis of pooled clinical trial data for sitagliptin and vildagliptin indicates an increased risk for infection (nasopharyngitis and urinary tract infection) and headache [31].
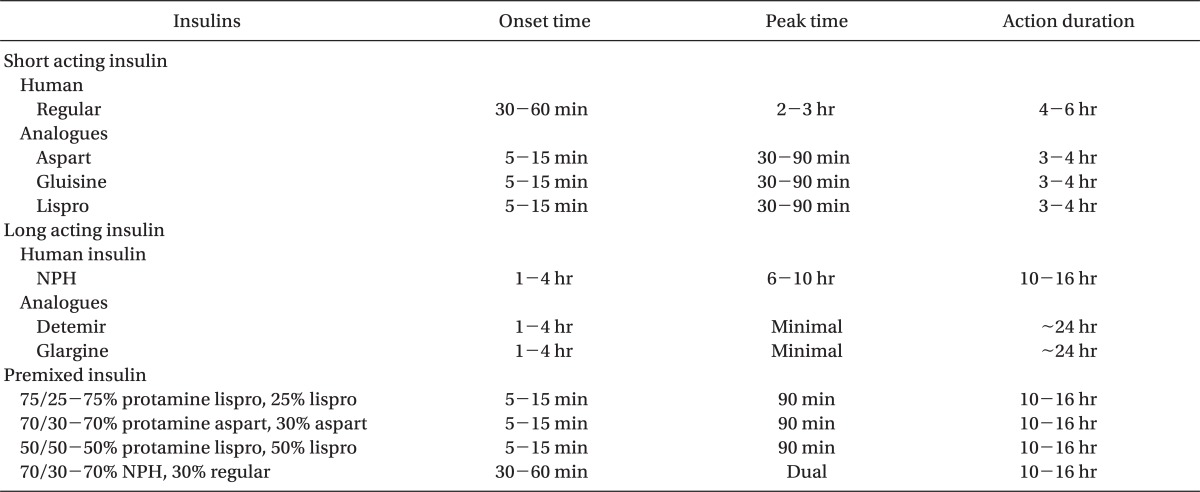
Insulin
Due to the progressive β-cell dysfunction that characterizes type 2 diabetes, insulin replacement therapy is frequently required [32]. Importantly, most patients maintain some endogenous insulin secretions, even in late stages of the disease. Starting insulin therapy with low doses in combination with oral agents is effective in the control of glycemic level and maintaining HbA1c values [33].
Insulins can be classified as short-acting or long-acting (Table 4). Short acting insulin analogues are lispro, aspart and glulisine. Insulin lispro is an insulin analogue, in which the 28th and 29th amino acids (lysine and proline) on the insulin B-chain have been reversed by recombinant DNA technology. Insulin aspart and insulin glulisine are other genetically modified insulin analogues with properties similar to lispro. These insulin analogues have full biologic activity, but fewer tendencies for self-aggregation, results in more rapid absorption and onset of action and a shorter duration of action [34]. Long acting insulin analogues are glargine and detemir. Insulin glargine is long-acting biosynthetic human insulin, which differs from that of normal insulin. Compared to the neutral protamine Hegedorn (NPH) insulin, it is released very slowly from the injection site and its duration of action is prolonged, allowing a relatively constant basal insulin supply without peak for more than 24 hours. A lower incidence of hypoglycemia, especially at night, has been reported with insulin glargine when compared to NPH insulin [33].
The principle of insulin use is the creation of as normal a glycemic profile as possible without unacceptable weight gain or hypoglycemia. As initial therapy, unless the patient is markedly hyperglycemic and/or symptomatic, basal insulin is typically added [35]. Basal insulin provides relatively uniform insulin coverage throughout day and night, which mainly controls blood glucose by suppressing hepatic glucose production in between meals and during sleep. Either intermediate-acting (NPH) or long-acting (glargine or detemir) formulations may be used. Although the majority of patients with type 2 diabetes requiring insulin therapy can be successfully treated with basal insulin alone, because of progressive diminution in their insulin secretory capacity, some will require prandial insulin therapy with shorter-acting insulins. Newer fast acting analogues, such as insulin lispro, insulin aspart, or insulin glargine, have a rapid onset of action and 4-5 hours of action duration that is normal meal time peaks of plasma insulin more closely than human regular insulin, therefore, immediate post-prandial hyperglycemia and late post-prandial hypoglycemia is rare [33].
There have been recent reports suggesting increased risk of cancer (colon, pancreas, breast and liver cancer) in patients taking long acting insulins. Cancers linked with DM are also associated with obesity or insulin resistance, so the cause may be multifactorial [36].
Anesthetic Consideration
According to the increasing diabetic population worldwide, anesthesiologists have a more chances to encounter patients with DM in the management of perioperative period. Nondiabetic patients may become hyperglycemic state due to a combination of tissue insulin resistance and decreased insulin secretion in the perioperative period. In diabetic patients, surgery and trauma are associated with an increase in the secretion of catabolic hormones in the presence of relative insulin deficiency. Therefore, the aim of perioperative metabolic management should be to avoid excessive hyperglycemia, hypoglycemia and loss of electrolyte such as potassium, magnesium and phosphate [37].
It is known that long-term intensive glycemic control in type 2 diabetic patient significantly reduced microvascular (retinopathy, nephropathy, neuropathy) complications [4,7]. In addition, control of hypertension and dyslipidemia is essential, blood pressure < 130/85 mmHg and a low density lipoprotein cholesterol level < 100 mg/dl are suggested a standard of care [38]. Observational study of 3,112 cases by Frisch et al. [39] showed that perioperative hyperglycemia is associated with increased hospital length of stay, hospital complications, and mortality after noncardiac general surgery. Especially, in this study, the rate of postoperative complications such as pneumonia, wound infection, urinary tract infection, acute myocardial infarction, and acute renal failure, was higher in diabetic patients than nondiabetics during the hospital stay. Tight glucose control in perioperative period has been recommended from a seminal study of 1,548 intensive care patients by Van den Berghe et al. [40], mostly post-cardiac surgery. In this study, tight glycemic control (blood glucose maintained in the range 80-110 mg/dl) showed decreased mortality rate than normal glycemic control (blood glucose maintained below 216 mg/dl), from 8.0% to 4.6% in patients who remained in intensive unit for over 5 days. Also, intensive insulin therapy in this study was associated with a decrease in the incidence of bloodstream infections, acute renal failure, polyneuropathy, and a decrease in the incidence of mortality from multi-organ failure. But, in this Belgian study, approximately 5% of the patients had hypoglycemic episodes despite the increased scrutiny of an intensive care. The other large observational study of Finney et al. [41] argued for maximal benefit in the range of 144 to 180 mg/dl. Therefore, even if these studies are postulated that insulin therapy is not harmful, and may even be beneficial, it is unclear what targets of glycemic control are appropriate. It is clear that tighter control necessitates more insulin included its potential harmful effects, and the risk for hypoglycemia increase.
Inadvertent hypoglycemia should be seriously considered in diabetic patients during the perioperative period, because hypoglycemia under anesthesia may be longer in duration and more severe than in patients who might manifest symptoms, such as tachycardia, sweating, pallor, light-headedness, restlessness, confusion, even unconsciousness, and frequent blood glucose estimations are essential [42]. New drugs, such as incretin and amylin analogues, may show hypoglycemia as an adverse effect, particularly, if used in conjunction with an insulin preparation or sulfonylurea [28,43]. Decreasing the dose of pre-meal insulin or sulfonylurea may need to avoid hypoglycemia. These adverse effects may occur particularly in the first 4 weeks of treatment, and declines over time. These new agents have only recently appeared in clinical practice, consequently, adverse effects in anesthetized or surgical patients may not been reported. New long acting insulin, such as glargine and detemir, shows good glycemic control between meals without the risk of hypoglycemia, so they can be used as a basal insulin during the perioperative period without stopping before the day of surgery [44].
Nausea is the most common adverse effect associated with incretins and amylin pathways. Nausea is generally mild to moderate, and vomiting is less frequent, most prevalent in the early state of the treatment, in the 4-8 weeks, after than generally declines. However, vomiting still occurs in approximately 17% of the patients treated with exenatide [28]. Although there are no published reports of postoperative nausea and vomiting attributed to incretin and amylin pathways, it seems reasonable to expect that patients treated with these agents may experience more severe, or frequent postoperative nausea and vomiting. Therefore, it seems logical to withhold these agents in the perioperative period to reduce the likelihood or intensity of postoperative nausea and vomiting. Future clinical studies may provide evidence based recommendations of these agents for the surgery and anesthesia.
Delaying gastric empting by incretin peptides and amylin pathways is one of the mechanisms of these agents that decrease postprandial glucose levels. Gastroparesis is a feature of advanced DM and these agents that slow gastric empting may exacerbate this problem [45]. Incretin and amylin pathways seem reasonable to expect an increased risk of aspiration with these agents during the perioperative period, especially, those patients with peripheral neuropathy and gastroparesis as manifestation of DM.
Conclusion
As the newer treatments for DM become increasingly prevalent in clinical practice, clinical experience accumulates with these agents in anesthetized patients, and more definitive recommendations may be provided. Anesthesiologists should have a concerns based on the physiology and pharmacology of the currently used novel therapies for the treatment of DM. Also, the adverse effects and interactions of new agents that need more clinical experiences should be considered for the diabetic patients during the perioperative management.