Hypotension in patients administered indigo carmine containing impurities -A case report-
Article information
Abstract
Indigo carmine has been used for eight decades with few adverse effects. Several of our patients, however, experienced severe hypotensive episodes after indigo carmine administration within a period of one month. Analysis of the raw materials used to formulate the preparation of indigo carmine we used showed that they contained impurities. Following recall of these impure materials, none of our patients experienced further hypotensive episodes.
Indigo carmine is a blue dye widely used in urologic and gynecologic procedures to identify ureteral patency. Although biologically inert, it occasionally causes side effects including nausea, hypertension [1], bradycardia [2], and supraventricular bigeminy [3]. Severe hypotension after indigo carmine injection is extremely uncommon, with only approximately 10 cases reported in the literature [4-8]. Few of the patients in our institution have experienced adverse reactions associated with indigo carmine administration, with most of these being mild hypertension and bradycardia. Recently, however, within a one month period, five of our patients experienced severe hypotension after administration of indigo carmine. We herein describe these patients and review the literature on causes of adverse reactions.
Case Report
A 69-year-old woman with a transitional cell carcinoma in the renal pelvis underwent a laparoscopic nephroureterectomy. The patient had a history of hypertension, and had no history of allergy or previous exposure to indigo carmine. Anesthesia was induced with thiopental 400 mg and vecuronium 10 mg, and maintained with 2% sevoflurane after tracheal intubation. A 20-gauge catheter was inserted into her left radial artery to monitor continuous arterial pressure. During the operation, the patient's vital signs were stable, with blood pressure (BP) 100-130/70-95 mmHg, heart rate 62-88 bpm, and SpO2 98-100%. Two hundred minutes after the induction of anesthesia, she received a bolus injection of 40 mg indigo carmine (sodium indigotindisulfonate; Korea United Pharm., Seoul, Korea). One minute later, her BP suddenly decreased from 120/85 mmHg to 50/30 mmHg, but heart rate remained at 80-90 bpm. There was no evidence of massive blood loss or electrocardiographic changes prior to the hypotensive episode, and there were no skin rashes, angioedema, or any sign of bronchospasm during the hypotensive episode. She was immediately administered ephedrine 20 mg and phenylephrine 0.3 mg, which restored her BP to 120/75 mmHg. Postoperatively her vital signs remained stable and she was discharged without any sequelae. Ten days later, a 73-year-old man who underwent a radical retropubic prostatectomy under general anesthesia experienced severe hypotension after administration of indigo carmine. Ninety minutes after anesthesia induction, he received an injection of 40 mg indigo carmine, and then BP decreased from 110/65 mmHg to 60/35 mmHg without any skin rash or urticaria. He was administered ephedrine 40 mg and phenylephrine 0.3 mg, which restored his BP to the pre-indigo carmine level. The patient's postoperative course was uneventful.
Following these adverse events, we decided to dilute indigo carmine in normal saline and to administer it slowly over five minutes. Two days later, a 64-year-old woman underwent a partial nephrectomy. The patient had a drug allergy to Saridon-A (Roche Korea, Seoul, Korea), which contains acetaminophen, anhydrous caffeine, and isopropylantipyrine. Two hundred minutes after induction, she was administered 40 mg indigo carmine diluted in 20 ml of saline over five minutes. A sudden drop in BP was observed; from 125/72 mmHg to 45/30 mmHg, and injection of ephedrine 30 mg restored her BP within three minutes.
Following this third episode of hypotension, we suspected a manufacturing error and checked the lot number of indigo carmine. We discarded all of the indigo carmine with lot number 001, used in our third patient, and decided to use another lot number, 002. Two weeks later, two additional patients experienced severe hypotension following administration of indigo carmine. The first, a 68-year-old man, underwent transurethral bladder tumor resection under general anesthesia. Eighty minutes after induction of anesthesia, he was administered indigo carmine. Five minutes later, his BP dropped from 128/75 mmHg to 65/43 mmHg, and injection of phenylephrine 0.3 mg restored his BP. Another patient was a 77-year-old man who underwent transurethral resection of the bladder. Spinal anesthesia was induced with hyperbaric bupivacaine 11 mg at the L4-5 level. After forty-five minutes, he received indigo carmine, and then his BP decreased from 110/65 mmHg to 60/35 mmHg. After administration of 15 mg ephedrine, his vital signs remained stable throughout the rest of the procedure. Both patients were discharged postoperatively without any particular sequelae.
Following these episodes, we asked the manufacturer about the indigo carmine we had used. The company's director informed us that lot numbers 001 and 002 were manufactured with the same raw materials, and the manufacturer provided us with indigo carmine of lot number 906, which to date has not been associated with any such adverse events. The raw materials used to manufacture indigo carmines were analyzed, including their crystalline status, thermo-physiochemical properties and particle size distribution, all of which are required analytical criteria for drug manufacture, as determined by the Korea Food and Drug Administration. A comparison of the raw materials used to manufacture lot numbers 002 and 906 showed no differences. Further analysis of the raw materials by liquid chromatography (LC) and mass spectrometry (MS) showed that, when compared with lot number 906, lot number 002 had a separated peak on LC and an abnormal fragment peak on MS, suggesting the presence of impurities (Fig. 1). Based on these analytic results, all indigo carmine numbered as 001 and 002 were recalled and its usage was restricted.
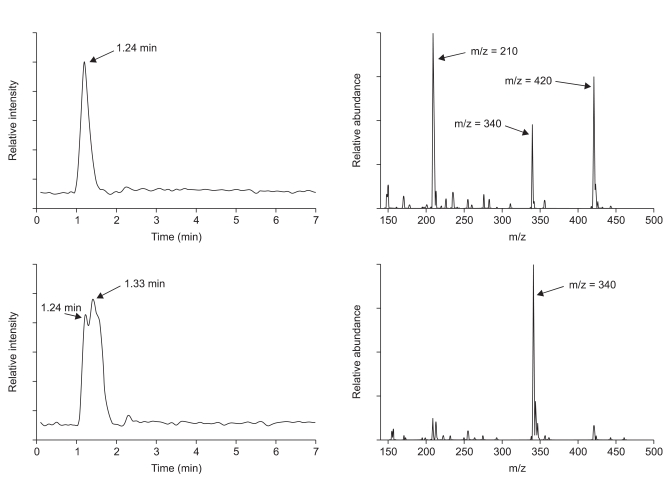
Analytic results of liquid chromatography (left) and mass spectrometry (right). Genuine raw material used to manufacture indigo carmine (top) shows a single peak at 1.24 minutes in liquid chromatography and this peak shows three mass peaks representing mass fragment of indigo carmine in mass spectrometry. The raw material for the indigo carmine that caused the hypotensive episodes (bottom) shows bimodal curve on liquid chromatography. The peak at 1.24 minutes in liquid chromatography shows the same three mass peaks as genuine raw material (not shown). A second liquid chromatography peak, at 1.33 minutes, shows abnormal relative abundance value, m/z = 340, by mass spectrometry. This peak is likely due to a chain of SO3Na that came off one side of the indigo carmine structure.
Discussion
The term anaphylaxis has been used for all types of acute life-threatening, generalized hypersensitivity reactions due to abnormal sensitivity to a triggering agent [9]. Although indigo carmine has no known activity in the human body, it has been found to directly activate alpha-adrenergic receptors, which may lead to reflex bradycardia and/or hypertension [10]. Hypotension after indigo carmine administration is an unexpected adverse effect and has been reported in fewer than 10 patients over 80 years of use.
Sudden, severe hypotension after indigo carmine administration was first reported in a series of four patients with no known drug allergies who underwent radical prostatectomy under epidural anesthesia [5]. Due to the temporal association between indigo carmine injection and the hemodynamic change, an idiosyncratic drug reaction was considered the most plausible cause. Usually, indigo carmine might cause both stimulant and depressant effects simultaneously, however, those cases could be induced by unopposed depressant component under sympathetic blockade [11]. Severe hypotension, bronchospasm and urticaria after administration of indigo carmine were observed in a patient under general anesthesia [7]. That patient, who was undergoing transurethral resection of a bladder tumor, manifested the complete spectrum of anaphylaxis five minutes after injection of indigo carmine, including hemodynamic collapse, cutaneous changes, laryngeal edema and bronchospasm. Due to a lack of prior allergies or prior exposure to indigo carmine, their case was thought to be caused by histamine release such as an anaphylactoid reaction. Life-threatening adverse reaction associated with indigo carmine presenting as even asystole was reported [12]. Severe hypotension, bradycardia, hypoxia were observed after indigo carmine injection, and then the patient became asystolic. Following chest compression and intraaortic balloon pump support, the patient's blood pressure was stabilized. The patient's cardiac arrest event was believed to be due to an anaphylactoid reaction in response to indigo carmine. A diagnosis of anaphylaxis requires evidence of involvement of three or more organs and the detection of specific IgE or a positive skin test to the alleged agent. However, since these reports did not verify these findings, it would be reasonable to diagnose these events as idiosyncratic reactions, including anaphylaxis.
Compared with the previous reports, our patients had somewhat bizarre clinical manifestations. During the period described for all five patients, we used lot numbers 001 and 002 of indigo carmine on 15 patients. However, hypotensive episodes requiring vasopressor occurred in only five patients. Hypotensive episodes in some of the other patients may have been overlooked because their symptoms were mild and they recovered immediately. In all five patients described above, saline replacement was adequate and there was no massive blood loss. There were no symptoms or signs suggesting anaphylactoid reactions, such as cutaneous changes or wheezing. Two patients who experienced hypotensive episodes were tested for hypersensitivity to indigo carmine of lot number 002 by skin prick tests and intradermal skin tests, and both were negative. Although a negative response to a suspected agent is unreliable in ruling out drug hypersensitivity [13], the clinical manifestations of these patients indicate that drug hypersensitivity or anaphylaxis was unlikely causes.
We focused on the five patients who experienced hypotension serially within a one month period, and suspected that flaws in production were responsible for these adverse reactions. During the manufacturing process, indigo carmine molecules may be fragmented by accelerated electron beams, and these fragmented ions can be classified by their mass to charge (m/z) ratios on MS (Fig. 2). Analysis of the raw materials by LC/MS showed an abnormal peak in lot number 002 that was not present in lot number 906. This additional peak contained abnormal amounts of fragmented ions, with m/z = 340, which had not been filtered out during the manufacturing process. These impurities may have affected hemodynamic response after administration of indigo carmine.
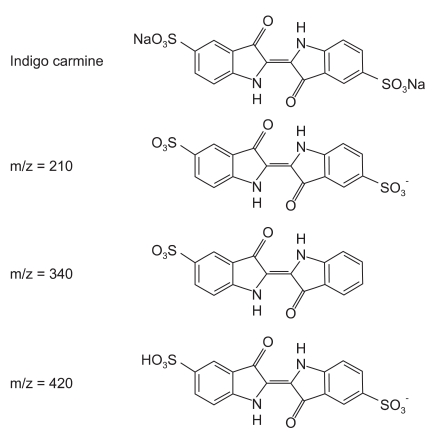
Structure of indigo carmine and its by-products during the manufacturing process. The term m/z represents mass to charge ratio, indicating the relationship between mass of an ion and its elementary charges.
Raw materials, reagents, and degradation during manufacture and storage are all possible sources of impurities in drugs [14]. These impure drugs may cause various adverse reactions, regardless of their pharmacological effects. Determining the lot number of the drug may be helpful in investigating the causes of adverse reactions. Usually, physicians tend to ignore or miss symptoms and signs if they are not critical. However, practitioners should always pay attention when using drugs and prevent further adverse drug reactions by meticulous monitoring and prompt reporting.
Acknowledgements
We would like to thank professor Tae-Bum Kim (Chief of the Asan Pharmacovigilance Center, Department of Allergy and Clinical Immunology, Asan Medical Center, University of Ulsan College of Medicine) for consultation about allergic skin tests and the reporting of adverse drug reactions.