Risk and crisis management in intraoperative hemorrhage: Human factors in hemorrhagic critical events
Article information
Abstract
Hemorrhage is the major cause of cardiac arrest developing in the operating room. Many human factors including surgical procedures, transfusion practices, blood supply, and anesthetic management are involved in the process that leads to hemorrhage developing into a critical situation. It is desirable for hospital transfusion committees to prepare hospital regulations on 'actions to be taken to manage critical hemorrhage', and practice the implementation of these regulations by simulated drills. If intraoperative hemorrhage seems to be critical, a state of emergency should immediately be declared to the operating room staff, the blood transfusion service staff, and blood bank staff in order to organize a systematic approach to the ongoing problem and keep all responsible staff working outside the operating room informed of events developing in the operating room. To rapidly deal with critical hemorrhage, not only cooperation between anesthesiologists and surgeons but also linkage of operating rooms with blood transfusion services and a blood bank are important. When time is short, cross-matching tests are omitted, and ABO-identical red blood cells are used. When supplies of ABO-identical red blood cells are not available, ABO-compatible, non-identical red blood cells are used. Because a systematic, not individual, approach is required to prevent and manage critical hemorrhage, whether a hospital can establish a procedure to deal with it or not depends on the overall capability of critical and crisis management of the hospital.
Hemorrhage is the No. 1 killer in the operating room all over the world. The Japanese Society of Anesthesiologists (JSA) started an annual survey of anesthesia-related critical events in the operating room in 1992. JSA sends confidential questionnaires to JSA-certified training hospitals, and then collects and analyzes the responses. Cardiac arrest as well as severe hypotension, severe hypoxemia, and other events that might develop into cardiac arrest or permanent disability of the central nervous system are registered. In this survey between 2004 and 2008 (n = 5,235,940), cardiac arrest was found to develop in 4.38/10,000 anesthetics and death within 30 postoperative days following intraoperative critical events was found to occur in 6.85/10,000 anesthetics [1]. Hemorrhage was responsible for 33% of cardiac arrests and 47% of deaths. Although two-thirds of the cases of hemorrhagic death occurred in emergency operations that were undertaken to save the lives of bleeding patients, one-third of them were caused by surgical procedures. Supplemental surveys concerning critical hemorrhage in the operating room that were also conducted by JSA between 2003 and 2005 revealed that hemorrhagic events were complicated by many human factors and then developed into critical hemorrhage [2]. Taking the results of another survey by JSA into consideration, blood loss exceeding 5,000 ml developed in 15 patients a day in the operating rooms of JSA-certified training hospitals, critical hemorrhage developed in 2.6 patients a day, and hemorrhagic death occurred in one patient a day.
In this review, I will discuss how we can prevent critical hemorrhage and how we can deal with those cases caused by surgical procedures in particular. Each surgeon and each anesthesiologist were partly responsible for critical hemorrhage. However, what we need is a systematic approach. We have to eradicate sources of error as much as possible.
Critical Hemorrhage
Rapid loss of large amounts of blood
Massive hemorrhage can be defined as follows: (i) blood loss exceeding circulating blood volume within a 24-hour period, (ii) blood loss of 50% of circulating blood volume within a 3-hour period, (iii) blood loss exceeding 150 ml/min, or (iv) blood loss that necessitates plasma and platelet transfusion [3], although there is no universal definition. However, we should note that critical hemorrhage can occur even though the hemorrhage does not meet the above criteria. Surveys concerning critical hemorrhage caused by surgical procedures (n = 1,105), which were conducted by JSA between 2003 to 2005, revealed that 63% of patients had blood loss of more than one circulating blood volume, and that 37% of them had blood loss of more than two circulating blood volumes. They also revealed that 51% of patients had blood loss exceeding 120 ml/min, and that 31% of them had blood loss exceeding 240 ml/min, although maximum hemorrhagic speed was not reported in 22% of patients [2]. The results clearly showed that we have to consider the volume and the speed of blood transfusion to save a patient's life. The results also suggested that blood loss of under one circulating blood volume or 120 ml/min was responsible for critical hemorrhage, indicating that blood loss that does not meet a general definition of massive hemorrhage can threaten patients in the operating room: some factors other than the volume or the speed of blood loss threatened surgical patients. Patient's ischemic co-morbidity, preceding anemia, delay of blood transfusion, and delay of blood supply may make hemorrhage critical regardless of blood loss.
Human factors affecting hemorrhagic critical events
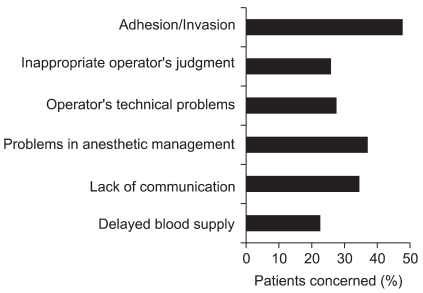
Anesthesiologists evaluated factors affecting hemorrhagic critical events caused by surgical procedures as shown in Fig. 1 [2]. Adhesion or invasion of diseased tissues is usually judged to be unavoidable. However, failure to foresee adhesion or invasion might be responsible for hemorrhage. While foreseeing these factors is impossible, unreasonable lysis of adhesion or resection in circumstances where red blood cells (RBCs) are not readily available might be responsible for critical hemorrhage.
Surgical problems include inappropriate judgments (27%), poorly developed skills (26%), delay of recognizing bleeding during endoscopic procedures (3%), and incorrect handling of surgical devices (3%). When critical events develop in the operating room, communication among the staffs concerned is important to avoid exacerbation of critical conditions caused by hemorrhage and to minimize the adverse effects of massive hemorrhage on patients. However, communication between surgeons and anesthesiologists seemed to be poor in 34% of the cases.
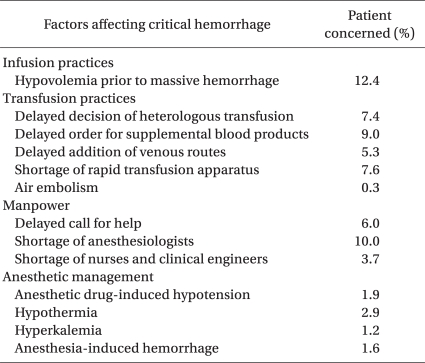
Details of anesthetic management factors responsible for critical events are listed in Table 1. These include hypovolemia just prior to bleeding (12%), shortage of supportive anesthesiologists (10%), delay in ordering additional blood products (9%), shortage of rapid infusion/transfusion apparatus (8%), delayed judgment to start blood transfusion (7%), and delayed addition of venous routes (5%). Hypovolemia prior to bleeding might be caused by restricted fluid management [4] and the difficulty of evaluating intravascular volume. Delayed judgment to start blood transfusion might be caused by a basic policy to avoid allogeneic blood transfusion because of its infectious and immunosuppressive effects [5], underestimation of blood loss due to bleeding into intra-abdominal/intra-thoracic/intra-vaginal cavities, or the absence of monitoring devices to measure hemoglobin levels continuously. It is unclear whether allogeneic blood transfusion during the perioperative period might affect long-term survival after surgery [6,7]. Difficulty to predict future blood loss and administrative pressure to avoid ordering too many blood products will cause delay in ordering additional blood products. Delay in ordering additional blood products, shortage of supportive anesthesiologists, and shortage of rapid infusion/transfusion apparatus cause delay in blood transfusion resulting in life-threatening hypovolemia and/or anemia. Because the maximal speed of blood loss exceeded 120 ml/min in about half of the cases of critical hemorrhage, maintaining multiple large-bore blood access is a prerequisite for saving a patient's life.
Anesthetic Management that Complicated Surgical Hemorrhage. Analysis of 1,105 Patients Who Developed Critical Hemorrhage due to Surgical Procedures
We anesthesiologists should be cautious when controlling anesthesia depth. Hypotension induced by anesthetic drugs affected critical events in 1.9% of patients developing life-threatening hemorrhage. More importantly, hypertension, bucking, and patient movement, which are manifestations of light anesthesia or shortage of muscle relaxants, were reported to induce massive hemorrhage. Therefore, maintaining adequate levels of anesthesia depth is important to avoid anesthesia-related or anesthesia-induced critical hemorrhage.
Problems of blood supply included delayed delivery of blood products from blood banks to hospitals (9%), exhaustion of time by cross-matching (8%), shortage of manpower during nights or weekends (6%), delayed delivery of blood products from blood transfusion services to the operating room (4%), and exhaustion of time by irradiation in blood transfusion services (4%). In Japan, the safe limit of time required for emergency delivery of blood products from blood banks to hospitals seemed to be between 30 and 45 min (unpublished data). Blood-type examination takes 9+/-7 min by manual procedures and 12+/-7 min by automatic procedures. Cross-matching takes 25+/-12 min by manual procedures and 29+/-10 min by automatic procedures [8]. The time required for blood-type examination and cross-matching might be longer during nights or weekends. We should also consider the following points: (i) withdrawing blood samples from a patient and then transporting them to blood transfusion services will take about 15 min, (ii) transfusing multiple trauma patients with ABO-identical RBCs instead of emergency O-type RBCs is associated with a higher risk of incompatible transfusion (wrong blood in tube) [9], and (iii) blood testing during nights or weekends by untrained staff can cause errors in blood-type judgment and in clerical work. Therefore, we should transfuse uncross-matched O-type RBCs in extreme emergencies until the blood-type is known, and then transfuse uncross-matched type-specific RBCs. The only situation in which we can transfuse fully cross-matched RBCs is when time permits. Manpower affects the outcome of hemorrhage or massive hemorrhage. It has been demonstrated that the mortality rate due to obstetric hemorrhage was significantly higher in hospitals with only 1 obstetrician than in those with 2 or more obstetricians [10]. The effects of manpower shortage easily become overt during nights or weekends, especially when a patient is in a severe condition. Overall survival from intraoperative massive hemorrhage during emergency surgery was not affected by time of day or day of week. However, when blood loss exceeded 200 ml/kg, the survival rate was significantly lower during nights or weekends than during day/evening (unpublished data). In Japan, half of hospitals with more than 500 beds purchase un-irradiated blood products. Because in-hospital irradiation takes between 5 and 15 min, it delays the delivery of blood products from blood transfusion services.
These human factors including adhesion or invasion were reported to be involved in 191% of 1,105 cases of critical hemorrhage caused by surgical procedures, indicating that multiple human factors are involved in critical hemorrhage [2]. This implies that drastic changes in patient care including changes in surgical procedures, anesthetic management, blood transfusion practices, and practices in blood transfusion services are required to save patients' lives.
We anesthesiologists should also be cautious of complications caused by massive and/or rapid transfusion. Hypothermia and hyperkalemia, both of which might cause cardiac arrest, have been reported to complicate 2.9% and 1.2% of patients with massive hemorrhage, respectively. Hypothermia might induce coagulopathy by inhibiting activation of coagulation factors as well as by inhibiting platelet aggregation. Although rapid infusion/transfusion apparatus is effective in massive/rapid transfusion, incorrect handling of the apparatus can cause massive air embolism. Air embolism by this mechanism was reported in 0.3% of patients with massive hemorrhage.
Emergency or massive transfusion practices: present status
We investigated the outcome of massive hemorrhage both in elective and in emergency patients, as well as the amount of blood stored in hospitals with more than 500 beds [11]. Fig. 2 shows that mortality rate increases with increasing blood loss and with decreasing minimum intraoperative hemoglobin level [11]. It should be mentioned that some patients whose minimum intraoperative hemoglobin level was maintained above 5-7 g/dl died following massive hemorrhage. The permissive limit of anemia has been reported to be 6 g/dl in a young healthy patient [12,13]. Some authors suggested that healthy patients were able to withstand a hemoglobin level of 5 g/dl in acute isovolemic hemodilution [14]. However, our investigation showed that maintaining hemoglobin level above permissive limits of anemia did not guarantee a good outcome. Possible explanations for this are as follows: (i) our study population might have had a low cardiopulmonary reserve or high oxygen consumption, and (ii) intravascular volume was coincidentally low due to rapid or massive hemorrhage. Complete examination of cardiopulmonary reserve is sometimes difficult, especially in an emergency setting. The rate of blood loss is unpredictable, and it is sometimes more rapid than RBC transfusion, resulting in deficits in intravascular volume and oxygen delivery. When solutions or blood products other than RBCs are rapidly infused or transfused to counter rapid blood loss, dilutional anemia develops. Therefore, a margin of safety in hemoglobin level should be considered in massive hemorrhage. In the British guidelines on massive hemorrhage, maintaining hemoglobin levels of more than 8 g/dl is recommended, while it is stated that the general indication of RBC transfusion is hemoglobin levels of less than 6 g/dl [13]. We should also be aware that hemoglobin level is a poor indicator of blood loss in acute situations [13].

Outcome of 1,257 patients whose intraoperative blood loss exceeded 5,000 ml in terms of the function of blood loss (A) and the minimum intraoperative hemoglobin level (B).
In the 1,257 patients whose intraoperative blood loss exceeded 5,000 ml, median, 75th percentile, and 90th percentile of amount of RBC transfusion were 20, 30, and 50 units (one unit corresponds to 200 ml of whole blood in Japan), respectively [11]. Meanwhile, the mean stored amount of RBCs was as follows: O-type, 11 units; A-type, 12 units; B-type, 8 units; and AB-type, 6 units [11]. The ratio of population of O-, A-, B-, and AB-type in Japan is 4 : 3 : 2 : 1. Therefore, when blood loss exceeds 5,000 ml, the stored amount of ABO-identical RBCs does not meet the requirements in many hospitals, even those with more than 500 beds, indicating that emergency delivery of ABO-identical RBCs from blood banks and/or transfusion of ABO-compatible, non-identical RBCs are required. We investigated 2,597 patients between 2006 and 2008, whose blood loss exceeded 5,000 ml and blood type was other than O-type. The overall mortality was 19%, while uncross-matched ABO-identical and ABO-compatible, non-identical RBCs were transfused in only 9% and 1.7% of patients, respectively. In 407 patients whose minimum intraoperative hemoglobin level was less than 5 g/dl, mortality was 37%, while uncross-matched ABO-identical and ABO-compatible, non-identical RBCs were transfused in only 20% and 5.4% of patients, respectively. In 164 patients who required cardiac massage in the operating room, mortality was 73%, while uncross-matched ABO-identical and ABO-compatible, non-identical RBCs were transfused in only 20% and 7.9% of patients, respectively.
Hemolytic reactions due to emergency transfusion practices
What disturbed emergency blood transfusion practices in the operating room or in blood transfusion services? The background is that the Ministry of Health, Labor, and Welfare has strongly recommended ABO-identical blood transfusion in order to prevent incompatible blood transfusion. The 4th edition of the guidelines for practice of transfusion established in 2009 by the Ministry of Health, Labor, and Welfare states that, in an emergency, "ABO-compatible, non-identical RBCs are permitted to be transfused in exceptional circumstances". In these public guidelines, the fundamental concept that saving the patient's life is the priority in blood transfusion for critical hemorrhage is lacking. As a consequence, medical practitioners have not been educated about emergency transfusion practices. Indeed, 40% of hospitals with more than 500 beds reported that there were some impediments that disturbed ABO-compatible, non-identical RBC transfusion [12]. The major impediment was insufficient consensus among medical staff, followed by safety issues and hesitation among medical staff including anesthesiologists.
What is the level of safety of uncross-matched ABO-identical or ABO-compatible, non-identical RBC transfusion? If the antibody screen is negative, type-specific uncross-matched blood will result in a hemolytic reaction in less than 1/50,000 units, which is lower than the reported incidence of delayed hemolytic reactions in the ordinary setting [5]. In Japanese patients who are not registered in computer cross-matching, the incidence of delayed hemolytic reaction is estimated to be about 1%, because the possibility of RhD negativity is 0.5% and the possibility of carrying irregular antibodies that may cause hemolytic reactions including anti-RhE, anti-Fya&b, and anti-Jka&b antibodies is 0.5% in Japan. Indeed, FDA reported 25 fatal hemolytic reactions probably due to irregular antibodies between 2005 and 2008, although whether uncross-matching was responsible was not mentioned [15].
It has been reported that 100,419 units of emergency O-type RBC transfusion during the Vietnam War did not cause lethal complications, while 24 patients who were transfused with cross-matched blood or type-specific blood exhibited lethal hemolytic reactions during the same period owing clerical error [9]. Dutton et al. [16] have summarized 4 reports and their own results concerning the safety of uncross-matched O-type RBCs in civilian trauma patients. They analyzed 790 patients or 2,041 units of RBCs and did not find severe or lethal complications, although at least 13 patients had transient alloimmunization. These low rates of complications might be partly explained by immunosuppression that is associated with massive hemorrhage or hemorrhagic shock. The other explanation is that transfused RBCs are lost from the circulation so rapidly that they cannot induce immune reactions.
The incidence of acute hemolytic reactions mainly due to major mismatch has been reported to be 1/25,000-50,000 units [5]. The Japanese Society of Transfusion Medicine and Cell Therapy reported that the incidence of major mismatch is 1/200,000 bags, of which about one-third of cases were related to RBC transfusion.
Therefore, we can speculate that the incidence of lethal hemolytic reactions associated with uncross-matched O-type RBCs is very low, and the incidence of delayed hemolytic reactions in patients whose irregular antibody is not examined is about 1%. On the other hand, the mortality rate is 19% when blood loss exceeds 5,000 ml. If hemorrhage is associated with severe anemia, critical events such as severe hypotension, or cardiac arrest, the mortality rate increases stepwise.
Risk-benefit analysis clearly shows that there is no rationale to abandon emergency transfusion practices to avoid associated complications.
Crisis Management
Declaration of emergency
Crisis management in critical bleeding resembles that in disaster medicine [17]. In order to save as many victims' lives as possible in major incident medical management, organization of many related staff is mandatory. The principal components of disaster medicine are summarized as CSCATTT: C for command, S for safety, C for communication, A for assessment, T for triage, T for treatment, and T for transport. If we replace S for safety of a bleeding patient in the operating room and T for transport of blood products from a blood bank to a hospital, we can adapt CSCATTT to actions against massive hemorrhage. The major problems underlying major incident medical management at the scene of an accident are reported to be a failure in communication as well as hesitation to declare an emergency.
To rapidly deal with critical bleeding in the operating room, effective communication among responsible staff is mandatory, especially between anesthesiologists and surgeons, between operating rooms and blood transfusion services, and between blood transfusion services and a blood bank. When critical bleeding occurs, a commander, a person in charge, is appointed. Senior physicians of the anesthesiology department or of the department in charge are candidates for commander. The first task for a commander is to carry out a medical assessment of the hemodynamic status of a patient and the most likely course of ongoing bleeding, and then to declare an emergency.
Guidelines for the management of critical bleeding in obstetrics published in 2010 by 5 related scientific societies including JSA proposed the urgency code of hemorrhage as shown in Fig. 3. This code was introduced to facilitate communication between the operating room and blood transfusion services. The idea is the same as a triage tag in disaster medicine, and staff other than doctors can easily understand the order of urgency. The urgency code clarifies what each medical staff should do according to bleeding and a patient's status. For example, when code I or code red is declared by a commander in the operating room, staff in blood transfusion services have to try their best to supply blood products including ABO-compatible, non-identical RBCs to the operating room as promptly and at as sufficient levels as possible. The process is dynamic, and coding is usually altered by further bleeding or after treatment. Because this urgency code should also be applied to facilitate communication between hospitals and blood banks in the near future, it is important to standardize the urgency code soon.

Urgency code for emergency blood transfusion. RBCs to be permitted differ among hospitals according to the amount of stock, manpower of the blood supply center especially during nights/weekends, and the time required for emergency blood supply from blood banks. Code color for I, II, III is red, yellow, green as in the case of a triage tag. *RBC: red blood cell.
Declaration of code I, an emergency, by a commander is also essential for calling out as many supportive medical staff including anesthesiologists, physicians of the department in charge, operating room nurses, and clinical engineers as possible and for correcting plans of surgical procedures and anesthetic management as well as infusion/transfusion practices. Keeping pre-operative plans of patient management will often provide catastrophic events. As mentioned in disaster medicine, the initial step of crisis management will be frustrated by reluctance to declare an emergency, failure in communication among medical staff including medical engineers in blood transfusion services, and a shortage of manpower. Professional pride, fear of criticism of calling many staff unnecessarily, or escape from the difficult situation can cause indecision of declaring code I. None of these is acceptable. Survival of the patient has to be prioritized in taking action against critical bleeding.
A commander comprehensively assesses hemodynamics, test data, the hemostatic condition, and blood product supply system, and then consults the surgeon regarding the continuation of planned surgery or change of surgical procedure. A commander has to keep in mind the following points: prompt decision-making, concrete as well as concise instructions, following the algorithm, watching for violations by medical staff, and confirmation of exact time recording. The other important task of a commander is to cancel an emergency, and to offer an opportunity to evaluate the validity of the responses later.
Blood Transfusion
Red blood cells
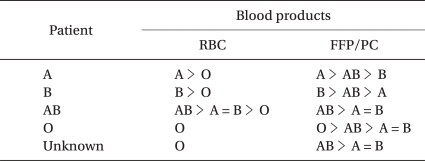
When the blood type is unclear, but time is short, O-type RBCs are used (Table 2). However, transfusion of ABO-compatible, non-identical RBCs and/or infusion of artificial colloid solution affect blood-type examination and cross-matching test, making it difficult to switch promptly to ABO-identical RBC transfusion. Therefore, before the start of ABO-compatible, non-identical RBC transfusion and/or artificial colloid solution, blood samples for blood-type examination and cross-matching should be taken, especially in an emergency outpatient.
When the blood type is clear, but time is short, the cross-matching test is omitted, and the same ABO-type blood is used. When ABO-identical RBCs are not available, ABO-compatible, non-identical RBCs are transfused. When the patient's blood type is AB, A- or B-type RBCs should be considered prior to O-type RBC transfusion (Table 2). For RhD-negative patients, the same ABO-type RhD-positive RBCs can be used on condition that anti-RhD antibody is absent. Even when a patient has an irregular antibody, ABO-compatibility is prioritized without performing a cross-matching test.
When a blood transfusion service supplies uncross-matched RBCs, its staff will start a cross-matching test thereafter. If the test reveals incompatibility, blood transfusion service staff should immediately report the result to operating room staff before the corresponding RBCs are transfused. In order to switch to the same ABO-type blood transfusion, the cross-matching test with newly collected fresh blood from the patient is performed by the saline method, and then ABO-identical RBCs are used.
Delayed hemolysis may occur several hours to 3 weeks after the completion of transfusion of irregular antibodies and anti-RhD antibody. Symptoms are severer the earlier reactions occur. When hemolysis develops, a febrile reaction and hemoglobinuria develop. Laboratory examination shows a decrease in hemoglobin level by more than 2 g/dl, an increase in LDH, an increase in indirect bilirubin level, positive direct globulin test, and positive cross-matching [5]. When hemolysis is confirmed, forced diuresis is induced by diuretics and infusion. Administration of anti-RhD immunoglobulin is considered when RhD-positive RBCs are transfused to an RhD-negative patient.
RBC salvage
When a large-volume RBC transfusion is necessary owing to massive hemorrhage, transfusion of autologous blood recovered from the surgical field is useful. When the blood loss is greater than 3 L, blood is aspirated, washed, and returned. The recovery rate of RBCs is about 40%. RBC salvage can minimize allogeneic RBC transfusion, including ABO-compatible, non-identical RBC transfusion.
In general, RBC salvage is thought to be contraindicated in patients undergoing resection of malignant tumors. Although infusion of malignant cells may cause their dissemination, salvaging RBCs is recommended to avoid hemorrhagic death, whether salvaged RBCs are transfused or not. RBC salvage is also thought to be contraindicated in patients undergoing Cesarean section because of the presence of fetal RBCs, fetal skin surface cells, and amniotic fluid. Taking into consideration the fact that hemorrhage is the leading cause of maternal death, RBC salvage should be considered after the delivery of fetus and placenta. Indeed, ASA recommended RBC salvage in an emergency even in Cesarean section [18]. An absolute contraindication of blood salvage is wound contamination.
Irradiation
Post-transfusion graft-versus-host disease is lethal. Even though RBCs and platelet concentrate (PC) are depleted of leukocytes, irradiation is mandatory. Therefore, irradiated blood products should be ordered when hospitals order supplemental blood products to a blood bank. It is also advisable that blood transfusion service start irradiation before the operating room orders supplemental blood products.
Massive and rapid transfusion
Multiple large-bore venous access is mandatory to treat critical hemorrhage. Central venous catheterization should be considered when required, even though central venous catheterization itself has some risks. Rapid infusion of cold blood products or rapid infusion of calcium through a central venous line should be avoided.
Rapid transfusion devices are useful to treat critical hemorrhage, although several precautions are required: (i) the indication has to be strictly complied with, (ii) only devices that are periodically and routinely maintained and inspected should be used, (iii) a person fully familiar with a device's operation must always be present and must take responsibility for using the device, (iv) air-detection and occlusion alarm has to be set at "ON" throughout use, and (v) particular attention must be paid to extravascular deviation of intravenous catheters. Warming blood products with the exception of PC is mandatory when the transfusion speed exceeds 50 ml/kg/hr.
Complications due to massive and rapid transfusion include metabolic changes (citrate intoxication, hyperkalemia, and hypothermia), dilution coagulopathy, and circulatory or iron overload.
Fresh frozen plasma
Massive hemorrhage is often associated with coagulopathy. Hemorrhage induces loss and consumption of coagulation factors. Infusion and transfusion of only RBCs cause dilution of coagulation factors [19]. Acidosis, hypothermia, as well as artificial colloid infusion inhibit coagulation even if levels of coagulation factors are adequate [3,20]. Recent evidence suggests that acute endogenous coagulopathy develops before coagulation factor depletion in injured victims [21]. Early traumatic coagulopathy develops in the presence of tissue hypoperfusion. In this context, the situation is quite different in elective surgery [20]. In elective surgery, trauma is controlled, normovolemia is maintained, and blood loss is replaced in a timely fashion.
Once coagulopathy develops, it increases blood loss. Because coagulopathy and blood loss make a vicious circle, prevention of coagulopathy is important. A general indication to transfuse fresh frozen plasma (FFP) is blood loss of more than one circulating blood volume or PT-INR greater than 2.0 [11]. However, in massive hemorrhage, British guidelines recommend that a therapeutic target of PT-INR should be less than 1.5, and that FFP should be given without PT-INR examination when blood loss is anticipated to exceed one circulating blood volume [13]. In such an emergency, we should also consider that thawing fresh frozen plasma will take about 30 min. Deficiency of fibrinogen develops earlier than that of any other coagulation factors: 100 mg/dl after loss of 120-170% of blood volume [3,5,22]. Since 5 units of FFP (400 ml) is equivalent to 1 g of fibrinogen, it increases the concentration by about 30 mg/dl in a person weighing 60 kg (circulating plasma volume: 3 L). Fibrinogen concentrate or cryoprecipitate is preferable when urgent treatment of coagulopathy is required, but volume loading is not desirable. Because PT-INR is not a sensitive parameter of fibrinogen deficiency, we have to directly measure fibrinogen levels. Another pitfall concerning PT-INR is that hypercoagulation develops in injured patients instead of the increase in PT-INR, which might cause pulmonary thromboembolism [23]. Thromboelastogrphy has been shown to be more sensitive to detect a hypercoagulable state [5,23].
Although off-label use of recombinant factor VIIa is reported to be effective in the treatment of massive hemorrhage in some settings, its efficacy and safety remain to be investigated [24]. It should be used after the completion of replacement of both platelets and coagulation factors, and when co-administration of tranexamic acid is contraindicated. Otherwise, use of recombinant factor VIIa may cause thrombotic complications such as myocardial infarction, stroke, and pulmonary embolism.
Platelet concentrate
Transfusion of platelets is ineffective until bleeding becomes surgically controllable. Platelets are transfused when the platelet count decreases below 50,000/mm3 or blood loss is exceeds ×1.5 blood volume. British guidelines recommend that platelet counts should be targeted to be above 75,000/mm3 to maintain them above 50,000/mm3 [13]. In a person weighing 60 kg, 10 units of platelets (containing 2 × 1011 platelets) are expected to increase the count by about 25,000/mm3.
Proactive administration of plasma and platelets
Massive transfusion practices in military trauma and then in civilian trauma have suggested that a 1 : 1 : 1 ratio of RBC : FFP : PC improves survival rate when a transfusion of more than 20 units (corresponding to 4,000 ml of blood) of RBCs is required within 24 h [25]. The idea is that the early identification and management of coagulopathy may help to better control traumatic hemorrhage and may reduce hemorrhagic mortality [21]. Whether the same concept can be applied to the elective surgical setting has yet to be elucidated. Because obstetric massive hemorrhage is usually associated with consumption coagulopathy, early management of coagulopathy is also required. It has been reported that the decrease in fibrinogen level is an early predictor of the severity of postpartum hemorrhage [26].
AB-type FFP/PC can be safely transfused irrespective of patients' ABO-blood type and of volume transfused. However, A-type and B-type FFP/PC can also be safely transfused irrespective of a patient's ABO-type (Table 2), unless transfusion volume does not exceed 2,000 ml. Transfusion of compatible, non-identical FFP and PC makes early treatment of coagulopathy possible.
Surgical procedures
A commander and a surgeon should discuss and decide surgical procedures: accomplishment of scheduled procedures, change to the minimum procedures, bimanual compression of the bleeding point, temporary packing [27], clamps of the artery or the aorta, or damage control surgery [28]. Hypothermia, acidosis, persistent hypotension, and shortage of blood products are the absolute indications for damage control surgery. After temporary wound closure with packing, improvement of the circulatory dynamics, coagulation system, oxygen transport ability, hypothermia, and acid-base equilibrium should be attempted in the ICU. Hemostasis by interventional radiology is also considered. In obstetric patients, prophylactic arterial embolization and catheterization have been reported [29].
Establishment of hospital actions
To rapidly deal with critical bleeding, not only cooperation between anesthesiologists and surgeons but also the linkage of operating rooms with blood transfusion services (transfusion division, laboratory test room, and others) and a blood bank are important. Related staff should have a thorough knowledge of the blood supply system of the hospital (blood transport and stock systems as well as time required for the procedures in blood transfusion services), the supply system of blood transfusion services, and the blood storage system in the operating room. It is desirable that the hospital transfusion committee prepares hospital regulations on 'actions to be taken against critical bleeding', and practices them in simulation. Once an emergency has been declared, the validity of surgical procedures, transfusion practices, and anesthetic management should be discussed in a morbidity and mortality conference, and re-evaluation of hospital actions should be carried out. It is also important to notify the actions as well as their background to all concerned staff.
The process by which the hospital transfusion committee prepares hospital regulations itself helps hospital staff to agree on emergency blood transfusion practices. Hospital actions should include the following: (i) agreement about assigning a commander and declaring an emergency, (ii) urgency code, (iii) emergency blood transfusion practices according to each urgency code (may differ depending on hospital size, stocks of blood products, activities of blood transfusion services during nights or weekends, time required for emergency supply of blood products from a blood bank), (iv) actions of blood transfusion services after supplying uncross-matched RBCs, (v) patient management after emergency blood transfusion, (vi) identification of patient and blood products (some computer-assisted collation systems released by manufacturers do not work in the case of emergency blood transfusion practices, and how to deal with multiple patients in the emergency room should be stated), (vii) irradiation, (viii) labeling the blood products to inform all related staff that emergency blood transfusion is going on, (ix) blood salvage, (x) off-label use of coagulation factor products, (xi) damage control surgery and intervention radiology, and (xii) informed consent.
A commander is requested to have sufficient clinical experience as well as the ability to make decisions appropriately and immediately, implying that he or she might experience strong mental stress. Hospital actions can minimize the deviation of aptitude as well as ability of a commander, and his or her mental stress. Hospital actions can also minimize hesitation of staff of blood transfusion services to deliver ABO-compatible, non-identical RBCs during nights or weekends. Hospital actions are meant to be followed as an organization, not as individuals, indicating that a person disobeying hospital actions is responsible for the resultant bad outcome of a patient. Therefore, hospital actions can eliminate antagonism between departments and with regard to seniority, which might delay or disturb emergency blood transfusion practices. Hospital actions also clarify the points and rules to be confirmed. By obeying hospital actions, misunderstandings and lapses can be eliminated, and everybody concerned can point out a commander's error. Establishing hospital actions enables a structured response against critical hemorrhage.
Centralization
In critical hemorrhage, a patient's life mainly depends on manpower and the availability of blood products. In terms of manpower, the quantity as well as the quality is important. Anesthesiologists, surgeons, radiologists, nurses, and clinical engineers in the operating room and staff of blood transfusion services are all essential to save a patient's life [10,11]. The survival rate after massive or critical hemorrhage might be lower in a hospital where surgeons can perform damage control surgery and radiologists can perform interventional radiology [27-29]. Even in JSA-certified training hospitals with more than 500 beds, there are insufficient ABO-identical RBCs to deal with blood loss exceeding 5,000 ml [11]. This implies that hospitals that perform risky procedures should be centralized. Table 3 shows the results of a JSA survey, which investigated 2,257 patients (1,281 elective and 633 emergency patients) with blood loss exceeding 5,000 ml. The results indicate that aortic surgery and liver transplantation should be centralized because of their high frequency among patients with massive hemorrhage, high incidence of massive hemorrhage, and high mortality rate. It seems that we should also pay attention to hepatic resection: the incidence of massive hemorrhage is low, but mortality after hemorrhage is higher than that of liver transplantation. The idea of centralization might be supported by the relationship between surgical volume and mortality [30,31]. To reduce hemorrhagic death, centralization of aortic and hepatic surgery should be prioritized.

Proportion, Incidence, and Mortality of Patients whose Blood Loss Exceeded 5,000 ml (from the Standpoint of Surgical Procedures)
In summary, many human factors are involved in the development of hemorrhage into critical events. Hemorrhagic mortality and morbidity in the operating room can be reduced by a systemic, not an individual, approach. Establishment of hospital actions to be taken to deal with critical hemorrhage depends on the overall capability of risk and crisis management of a hospital.