Acute, fatal postoperative myocardial infarction after laparoscopic cholecystectomy in a cardiac patient -A case report-
Article information
Abstract
This report presents the case of a 63-year-old man who had a myocardial infarction leading to coronary artery bypass graft 2 years earlier who subsequently underwent elective laparoscopic cholecystectomy. After an uneventful operation, the patient developed an acute postoperative myocardial infarction in the recovery room and died 19 days postoperatively. Anesthesiologists should be aware of the rare possibility of acute, fatal postoperative myocardial infarction and consider this complication when they perform the preoperative risk evaluation, anesthesia, and postoperative care for cardiac patients undergoing noncardiac surgery.
Postoperative myocardial infarction (MI) after noncardiac surgery is one of the most serious and prognostically ominous complications of surgery and anesthesia. Reported rates of postoperative MI in patients undergoing noncardiac surgery vary widely. While the incidence of postoperative MI in the general noncardiac surgical population is relatively very low (less than 1%) [1,2], the incidence is substantially higher in subgroups of patients with known cardiovascular risk factors and of older age [3].
We present a patient who underwent coronary artery bypass graft (CABG) 2 years earlier who had an acute postoperative MI in the recovery room after laparoscopic cholecystectomy.
Case Report
A 63-year-old man visited the emergency center of our hospital with epigastric pain and a fever. He was admitted for further evaluation and management. The diagnosis was gallbladder adenomyomatosis and he was scheduled for laparoscopic cholecystectomy (LC). He had hypertension and atrial fibrillation. He had experienced unstable angina, which led to stent insertion in the left anterior descending (LAD) coronary artery three times 12, 10, and 3 years earlier, respectively. Two years earlier, he underwent CABG due to MI. Coronary angiography performed just before the CABG showed severe triple vessel disease. His medical regimen at the time of admission for elective LC included: bisoprolol, nicorandil, cilazapril, aspirin, isosorbide mononitrate, fluvastatin, and diltiazem.
The preoperative electrocardiogram (ECG) on this admission showed nonspecific ST-T changes (Fig. 1A) and coronary angiography performed after this admission showed total occlusion from the proximal to the distal parts of the circumflex artery with intracoronary collaterals, diffuse 30% stenosis of the proximal and middle parts of the LAD with stents, 95% stenosis of the hypoplastic right coronary artery, and a patent left internal mammary artery to the obtuse marginal artery. The left ventricular function was preserved, with an ejection fraction of 0.59. The chest X-ray was within normal limits. His preoperative AST, ALT, hemoglobin and hematocrit were 48 IU/L, 87 IU/L, 13.2 g/dl and 36.9%. Other preoperative laboratory findings were within normal limits. He was premedicated with glycopyrrolate 0.2 mg and midazolam 1.5 mg. The patient repeatedly refused a treadmill test.
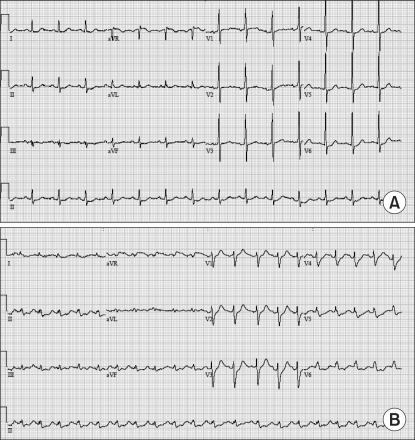
(A) The preoperative electrocardiogram (ECG) shows nonspecific ST-T changes. (B) The six-lead portable ECG shows sinus tachycardia and ST-depression in leads II, III, aVF, V5, and V6 when the patient complained of chest pain in the recovery room.
In the operating room, monitoring included a five-lead ECG with continuous ST segment analysis, pulse oximetry, noninvasive blood pressure, end-tidal CO2, esophageal temperature, bispectral index, and an arterial catheter placed in the left radial artery. General anesthesia was induced uneventfully with a bolus of etomidate 21 mg and rocuronium 50 mg. Alfentanil 500 µg was given at the time of intubation. Anesthesia was maintained with 2 L/min oxygen, 2 L/min nitrous oxide, and isoflurane with the concentration adjusted to maintain hemodynamic stability. Nitroglycerin (NTG) was given as a continuous intravenous dose of 0.5 µg/kg/min for coronary vasodilation. The patient was ventilated by a semiclosed circular circuit with a tidal volume of 700 ml and respiratory rate 12 breaths per minute. The blood pressure and pulse rate were stable throughout the operation at 130/80 mmHg and 80 beats/min, respectively. However, the ECG showed atrial fibrillation during the operation. Intraoperative intra-abdominal pressure (IAP) value of 13 mmHg was achieved by insufflating the abdominal cavity with a carbon dioxide (CO2). Intraoperative arterial blood gas analysis showed pH = 7.37, PaCO2 = 39.1 mmHg, PaO2 = 195 mmHg, HCO3- = 22 mEq/dl, Hb = 13.6 g/dl, Na = 135 mEq/dl, and K+ = 4.0 mEq/dl. The operation took 1 h and 5 min, and was uneventful. During surgery, he received 520 ml of replacement fluid and the urine output was 80 ml. He was subsequently transferred to the recovery room for further observation. The initial blood pressure in the recovery room was 130/80 mm/Hg and the three-lead ECG showed atrial fibrillation with 80 beats/min. In the recovery room, the patient still received the continuous NTG infusion that was started in the operating room.
Forty minutes after arriving at the recovery room, the patient complained of chest pain and dyspnea. His vital signs at that time were blood pressure 75/40 mmHg, heart rate 120 beats/min, and O2 saturation 85%. The ECG showed ST depression. He was intubated promptly; the NTG was stopped, and he was given an injection of phenylephrine 100 µg. The six-lead portable ECG (Fig. 1B) and cardiac markers were checked, and a central venous catheter was inserted in the left subclavian vein. The initial central venous pressure (CVP) value read 5 mmHg. We called a cardiologist and a chest surgeon. Soon afterward, the patient became asystolic on the ECG. Cardiopulmonary resuscitation (CPR) was begun immediately and epinephrine 1 mg and atropine 0.5 mg were administered initially and at 3-min intervals. Simultaneously, dopamine 20 µg/min/kg was started. During CPR, a portable transthoracic echocardiogram was obtained, which showed hypokinesia of the inferior, lateral, and posterior walls and akinesia of the anterior wall. The estimated left ventricular ejection fraction was 10%. The patient was transferred to the angiography room for insertion of an intra-aortic balloon pump (IABP). After the IABP was inserted, the patient's condition did not change. Immediately afterward, ECMO (Capiox Emergency Bypass System®, TerumoInc., Tokyo, Japan) was begun at flow rates between 2.8 and 4 L/min. Serum troponin-I values increased form 0.04 µg/L at the time of acute event in the recovery room to 12.52 µg/L 3 h later the event (normal range <0.05 µg/L). The left ventricular ejection fraction improved to 25%. Unfortunately, the patient died 19 days after surgery.
Discussion
Perioperative myocardial infarction is one of the most important predictors of short- and long-term morbidity and mortality associated with noncardiac surgery [4]. The American College of Cardiology and American Heart Association (ACC/AHA) practice guidelines recommend assessing patients' comorbidities and exercise tolerance, as well as the type of surgery to be performed, to determine the overall risk of perioperative cardiac complications [5]. Based on this assessment, selected patients should undergo provocative cardiac testing; others may require interventions.
In our case, a patient who underwent CABG because of a MI 2 years earlier was undergoing an elective LC. According to the risk of various types of noncardiac surgical procedures stratified by the ACC/AHA practice guidelines, elective LC, which is intraperitoneal surgery, is a surgical procedure at intermediate cardiac risk [5]. The reported cardiac risk of intermediate cardiac risk surgical procedures is generally less than 5%. However, during laparoscopic surgery, peritoneal insufflation to IAPs higher than 10 mmHg induces significant alterations of hemodynamics [6]. These disturbances are characterized by decreases in cardiac output, increased arterial pressures, and elevation of systemic and pulmonary vascular resistance. Especially, the decrease in cardiac output is proportional to the increase in IAP [7]. Anesthesiologists should consider the hemodynamic changes associated with laparoscopic operation during preoperative surgical risk evaluation of cardiac patients. Concerning the cardiac risk with a previous CABG, if the patient has had complete surgical revascularization in the previous 5 years, and if his or her clinical status has remained stable without recurrent signs or symptoms of ischemia in the interim, the likelihood of perioperative cardiac death or MI is extremely low [8]. Our patient had no recurrent signs and had preserved left ventricular function with an ejection fraction of 0.59, and was predicted to be at low cardiac risk because of the previous CABG.
The patient had hypertension and atrial fibrillation. Hypertension is an important risk factor for ischemic heart disease and results in increased systemic vascular resistance, decreased intravascular volume, and an exaggerated pressor response [9]. Furthermore, hypertension is one of the etiologies most frequently associated with atrial fibrillation (AF) [10], which can produce ischemia by increasing myocardial oxygen demand. In our case, the hypertension was well controlled during the preoperative and intraoperative periods, while the intraoperative and postoperative ECG showed AF.
Considering our preoperative evaluations, we regret that the patient's functional capacity was not checked with a treadmill test, although the patient refused the test to the last. Ergometric measurements on a treadmill inducing ischemia at low-level exercise [<5 metabolic equivalents (MET) or heart rate <100/min] identify a high-risk group, whereas the achievement of more than 7 MET (or heart rate >130/min) without ischemia identifies a low-risk group [11].
Another missed point of this case was the use of phenylephrine immediately after development of the symptoms considering low blood pressure and rapid heart rate. Phenylephrine is a selective α1-agonist and commonly used when peripheral vasoconstriction is needed and cardiac output is adequate, as in patients with coronary artery disease to increase coronary perfusion pressure without chronotropic side effects [12]. However, ejection fraction of him was decreased with hypokinesia. Anesthesiologists should consider the cardiac output state before use of drug and we thought that norepinephrine might be better than phenylephrine in our case or similar case.
Most ischemic episodes tend to start at the end of surgery and during emergence from anesthesia [13]. This period is characterized by increases in sympathetic tone and procoagulant activity. Increased sympathetic tone can result in increases in arterial pressure, heart rate, and cardiac contractility, and these conditions might lead to subendocardial ischemia by increasing myocardial oxygen demand and eventual myocardial infarction. In addition, the intense procoagulant activity and sympathetic stimulation in the postoperative period have been implicated in the development of coronary vasospasm, thrombosis, and the rupture of atheromatous plaque, leading to myocardial ischemia and infarction [4]. However, this mechanism is not very plausible because ST-depression is the most common ECG change and the classic ST-elevation marker of acute coronary occlusion is rare in the postoperative period [14]. Furthermore, postoperative MI has always been considered a relatively late complication, with the peak occurrence on the third day after surgery, and with an exceptionally high mortality of 30-70% [15].
In our case, ischemic symptoms of chest pain and ST-depression developed 40 min after surgery, while postoperative MI unusually occurs within 1 h after surgery in the recovery room. This case showed that postoperative MI can develop early after surgery, so anesthesiologists should cautiously monitor patients with coronary artery disease and cardiac problems in the recovery room. Other factors of concern for anesthesiologists-postoperative pain and physiological and emotional stress-may all combine to cause tachycardia, hypertension, increased cardiac output, and fluid shifts, which might result in subendothelial ischemia and eventual MI in high-risk patients [14]. Although patient controlled analgesia (PCA) was not applied routinely after LC in our hospital, in this case, PCA should be considered and might be helpful to the patient.
In conclusion, we described an acute, fatal postoperative MI in a cardiac patient with preserved left ventricular function after noncardiac surgery. Anesthesiologists must be aware that this rare complication could happen in cardiac patients after surgery. Anesthesiologists should always consider this complication when evaluating preoperative risk, performing anesthesia, and managing postoperative patient care, as well as the development of postoperative ischemic symptoms, including chest pain or ST-depression.