Sluggish decline in a post-transplant model for end-stage liver disease score is a predictor of mortality in living donor liver transplantation
Article information
Abstract
Background
The pre-transplant model for end-stage liver disease (pre-MELD) score is controversial regarding its ability to predict patient mortality after liver transplantation (LT). Prominent changes in physical conditions through the surgery may require a post-transplant indicator for better mortality prediction. We aimed to investigate whether the post-transplant MELD (post-MELD) score can be a predictor of 1-year mortality.
Methods
Perioperative variables of 269 patients with living donor LT were retrospectively investigated on their association with 1-year mortality. Post-MELD scores until the 30th day and their respective declines from the 1st day post-MELD score were included along with pre-MELD, acute physiology and chronic health evaluation (APACHE) II, and sequential organ failure assessment (SOFA) scores on the 1st post-transplant day. The predictive model of mortality was established by multivariate Cox's proportional hazards regression.
Results
The 1-year mortality rate was 17% (n = 44), and the leading cause of death was graft failure. Among prognostic indicators, only post-MELD scores after the 5th day and declines in post-MELD scores until the 5th and 30th day were associated with mortality in univariate analyses (P < 0.05). After multivariate analyses, declines in post-MELD scores until the 5th day of less than 5 points (hazard ratio 2.35, P = 0.007) and prolonged mechanical ventilation ≥24 hours were the earliest independent predictors of 1-year mortality.
Conclusions
A sluggish decline in post-MELD scores during the early post-transplant period may be a meaningful prognostic indicator of 1-year mortality after LT.
Introduction
Liver transplantation (LT) is usually has higher mortality rates than other types of surgery due to acute hepatic failure, graft size-related problems, ischemic time, original disease recurrence, cardiovascular complications, and infection [1-3].
Early determination of mortality factors are important for proper patient management using pre-transplant or intraoperative factors [4,5]. One prognostic marker is the model for end-stage liver disease (MELD) score, which was originally developed for predicting early mortality in patients waiting for LT [6]. Patients with higher MELD scores usually have more preoperative comorbidities and laboratory abnormalities, so pre-transplant MELD (pre-MELD) score can help predict pre-transplant fatal outcomes [7]. Despite controversy [8], the pre-MELD score is not generally a useful indicator of post-transplant mortality or in pre-transplant situations [9].
Patients after LT have much different physical conditions than pre-transplant periods. Graft-related problems cause more than 50% percent of late deaths [2]. MELD score is based primarily on liver-related laboratory findings such as bilirubin and international normalized ratio of prothrombin time (INR). Therefore, patients with changed liver conditions should receive a new MELD score. MELD score is partly driven by creatinine and can reflect post-transplant deterioration in renal function as well. Although pre-transplant renal dysfunction improves after transplantation [10], LT also leads to chronic renal disease, which can contribute to graft and patient survival [11].
Clearly, pre-transplant cross-sectional assessment of disease severity is important in predicting patient outcomes. However, re-evaluation or follow-up observation in the early post-transplant period may be more valuable for accurate prediction of long-term outcomes after surgery. Studies on post-transplant MELD (post-MELD) scores are rare, although it may be a predictor for short-term mortality [12,13]. An initially high MELD score in the immediate post-transplant period may decrease, increase, or remain unchanged according to recipient recovery patterns, which may also inform patient prognosis.
Although the MELD score system is invaluable and widely used for patients with end-stage liver disease for its convenience and simplicity, other prognostic indicators for critically ill patients in an intensive care unit (ICU) may be useful, such as acute physiology and chronic health evaluation (APACHE) II and sequential organ failure assessment (SOFA) scoring systems, which are used for mortality prediction from cirrhosis in the ICU patients [14-16]. Comparing the predictive ability of MELD scores and these ICU severity scores may be useful.
We therefore investigated whether changes in MELD scores or other ICU severity scores in the early post-transplant period were associated with mortality in LT recipients.
Materials and Methods
We collected perioperative and survival data in LT patients from November, 2003, to August, 2008, after approval of the Institutional Review Board at our university. Only adult (≥18 years) living donor LT cases were included. Transplantation was performed between the right hepatic lobes of both recipient and donor. Perioperative patient management was guided by routine LT protocols at our hospital. The electrical medical recoding system and chart system of the Transplantation Center at our hospital were used for this collection. The end point of this study was 1-year mortality after transplantation, and we studied the following variables.
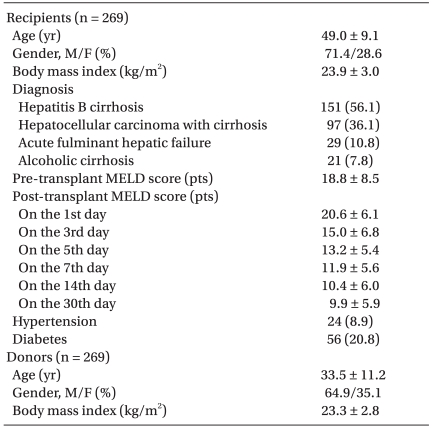
Recipient pre-transplant variables: age, Child-Pugh-Turcott (CPT) class C, MELD score, emergency surgery, heart disease history, hepatorenal syndrome, hepatic encephalopathy, ascites (≥1 L), alanine aminotransferease (ALT) level, and hyponatremia (Na+ < 130 mmol/L).
Recipient intraoperative variables: last lactate concentration, packed red cell transfusion, reperfusion syndrome, and surgery time.
Donor variables: age, graft macrosteatosis ≥20%, graft recipient weight ratio (GRWR).
Recipient post-transplant variables (within 7 days after surgery): reoperation, infection, pulmonary complication, low urine output (<1 ml/kg/h), prolonged mechanical ventilation (≥24 hours), APACHE II score, SOFA score on the 1st post-transplant day.
Post-MELD scores were calculated on the 1st, 3rd, 5th, 7th, 14th, and 30th post-transplant day, using the formula: R = 9.6 × loge (creatinine mg/dl) + 3.8 × loge (bilirubin mg/dl) + 11.20 × loge INR + 6.4, as reported by Kamath et al. [17]. Declines in post-MELD scores from the 1st day post-MELD score until the 3rd (D3), 5th (D5), 7th (D7), 14th (D14), 30th post-transplant day (D30) were also calculated.
Statistical methods
Predictive factors for mortality after LT were analyzed by Cox's proportional hazards regression model. Relative risks were calculated as proportional hazard ratio (HR) for each probable perioperative variable in a univariate model. Before multivariate analyses, selected continuous variables (P < 0.05) were dichotomized at their median, at clinically meaningful cutoff points, or at the point with maximal sensitivity and specificity for mortality by the analysis of area under the Receiver Operating Characteristic (ROC) curve. We performed multivariate analyses using a forward and backward stepwise Cox's regression model with the likelihood ratio test statistic. Results by multivariate analyses were displayed as HR, 95% confidence interval (CI), and P-value. Two side P values less than 0.05 were considered statistically significant. SPSS version 15.0 for Windows (SPSS Inc, Chicago, IL, USA) was used for most statistical analyses and ROC curve analysis was conducted using MEDCALC for Windows version 11.0 (MedCalc Software, Mariakerke, Belgium).
Results
Of 281 patients who underwent LT during the study period, 269 patients were included, with exclusions of 9 cadaver donations and 3 pediatric cases. Baseline demographics of LT recipients and donors are shown in Table 1. More recipients (11%) were older than 60 years than donors (1.5%). Recipients were predominantly male, and 56.1% had liver cirrhosis resulting from hepatitis B virus infection. Mean MELD scores of the recipients was 18.8 points at the pre-transplant period, and increased to 20.6 points just after transplantation. MELD scores then declined by more than 7 points until the 5th post-transplant day.
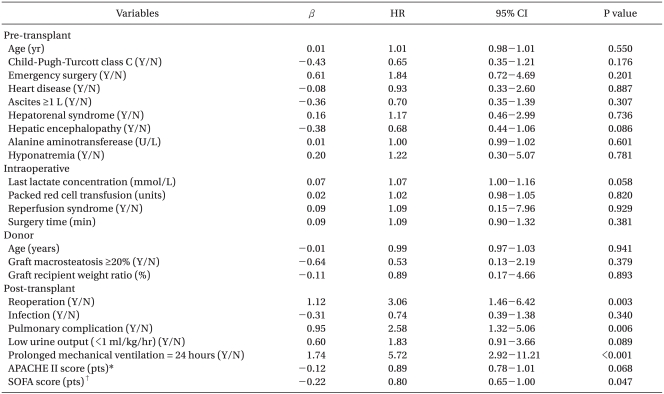
The 1-year mortality rate of LT recipients was 17.1% (n = 41). Eighteen (43.9%) deaths were associated with graft failure, and infectious complications such as pneumonia or sepsis were the second most common cause of death (n = 14, 34.1%). Cancer recurrence or metastasis (14.6%), cardiac problems (9.8%), bleeding (7.3%), suicide (4.9%), and renal failure (2.5%) followed as causes of death. The incidence of death cases with more than one cause of death was 26.8%, including multiorgan failure (7.3%). In univariate analysis, no pre-transplant, intraoperative, or donor variables were associated with mortality. Only post-transplant variables such as reoperation, pulmonary complication, prolonged mechanical ventilation ≥24 hours until the 7th post-transplant day, and SOFA score on the 1st post-transplant day were related to 1-year mortality after LT (P < 0.05, Table 2).
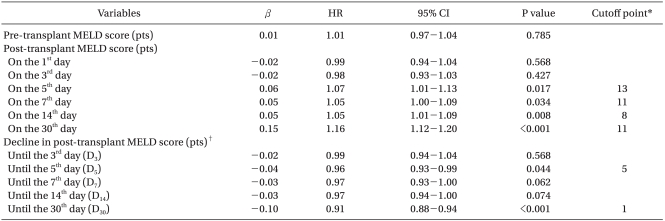
The association of MELD score related variables with mortality is displayed in Table 3. Pre-MELD and post-MELD scores on the 1st and 3rd day had no influence on 1-year mortality. After the 5th post-transplant day, all post-MELD scores had meaningful associations with 1-year mortality (P < 0.05). Changes in MELD score did not show a relationship with mortality. Only D5 and D30 were significant (P < 0.05), and could enter the next multivariate analyses. The cutoff point of D5 with the maximal combination of sensitivity and specificity for mortality by ROC curve analysis was 5 points.
Hazard Ratios for Mortality after Liver Transplantation According to Model for End-stage Liver Disease (MELD) Scores
Through multivariate analysis, we could establish a predictive model of mortality that included post-transplant prolonged mechanical ventilation ≥24 hours and decline in post-MELD score (Table 4). No post-MELD scores were significant in the final multivariate analysis. Instead, D5 less than 5 points was identified as the earliest independent predictor of 1-year mortality in living donor LT (HR 2.35, 95% CI 1.27-4.68, P = 0.007).

Predictive Model for Mortality after Living Donor Liver Transplantation by Multivariate Cox's Proportional Hazards Regression Model
Post-MELD declines reflect 1-year mortality, as shown in the survival graph (Fig. 1). Patients with D5 less than 5 points showed significantly lower 1-year mortality than patients with D5 higher than 5 points (74.1% vs 88.4%, P = 0.004).
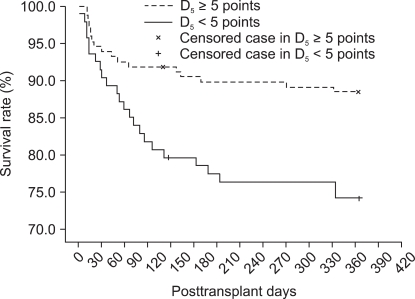
Comparison of survival rates between patients with and without D5 less than 5 points. The patients with D5 less than 5 points show a significantly lower 1-year survival rate than those without D5 less than 5 points (74.1% vs 88.4%; P = 0.004, by log-rank test in Kaplan-Meier analysis). D5: decline in post-transplant MELD score from the 1st day until the 5th post-transplant day.
Discussion
The MELD score was adopted for the organ allocation system in the United States in 2002, and was originally designed to assess the short-term mortality of patients with portal hypertension undergoing transjugular intrahepatic portosystemic shunt [6]. However, its simplicity to calculate and inclusion of important items have increased its use for predicting intraoperative or post-transplant outcomes as well as pre-transplant complications in patients who underwent LT. In addition to predicting the three month mortality of patients on LT waiting lists [18], a MELD score higher than 30 points indicates higher requirements for intraoperative transfusion and vasopressors [19,20]. However, pre-MELD score is not sufficiently reliable as a predictor of mortality [21,22]. This study also indicated pre-MELD score did not predict post-transplant mortality.
LT Patients have different pre-transplant and post-transplant situations. In particular, transplanted liver graft and immune suppression are critical for patient management and prognosis [23], with infection, recurrence of primary liver disease, and side effects of immunosuppressants playing critical roles in mortality [2,3,24]. Pre-transplant factors may help predict post-transplant mortality [4,25], but the survival of LT recipients depends on immunological or functional recovery of the liver graft.
Causes of mortality after LT are categorized as either graft-related or non-graft related. The main cause for graft-related mortality is recurrence of primary disease or hepatitis virus, and for non-graft related mortality is a cardiovascular event or de novo malignancy [2]. The riskiest time period for mortality is the first post-transplant year, particularly the first three months [26,27], with primary causes of mortality that include graft dysfunction, technical problems, and infection [28]. Graft-related deaths cause more than half of all mortality. Graft failure in cadaveric donor LT is influenced by donor age, donation after cardiac death, and split/partial grafts [29]. Living donor LT may have other risk predictors for graft failure, including small size discrepancy for metabolic requirements, or vascular problems resulting from thrombosis, or mechanical twisting because of smaller sizes. Liver graft evaluation and prediction system for early post-transplant may therefore be more useful than pre-transplant ones.
The MELD score is a good indicator of liver graft status because it measures bilirubin, INR, and renal function, and is simple to calculate. Pre-transplant renal dysfunction with estimated glomerular filtration rates <30 ml/min/1.73 m2 is often restored after LT, but immunosuppression frequently induces chronic kidney diseases [10]. Post-transplant kidney disease is a common complication after LT and impacts graft and patient survival [11,30]. A pre-MELD score ≥20 points had a higher incidence of post-LT chronic renal failure and higher mortality rate [31]. We therefore assessed post-MELD scores for comparison with other mortality indicators [32], and it was reliable in predicting short-term mortality at less than 90 days [12,13].
We showed that the post-MELD score could predict long-term mortality (1 year), but the pre-MELD score did not. Graft-related problems were the leading cause of death, so post-MELD score may reflect these graft-related problems. Renal failure did not contribute to death as much as we expected, and perioperative variables were not associated with mortality.
APACHE II scores, SOFA score, simplified acute physiology score (SAPS), and multiple organ dysfunction score (MODS) are ICU severity scoring systems used in mortality prediction [33,34]. In cirrhotic patients, SOFA score has been compared to other ICU severity scores, including APACHE II, failing organ systems (FOS), organ system failure (OSF), and liver specific scores including Child-Pugh-Turcott classification and MELD score [15,16]. SOFA was the most accurate predictor of mortality through analysis of the area under the ROC curves, but requires follow-up measurement. The score at 48 h after ICU admission or mean scores of multiple measurements are better than baseline scores on ICU admission day. APACHE II can also predict mortality in LT patients [14], although it tends to overestimate mortality rate in LT patients. We used 1-day post-transplant SOFA or APACHE II scores to capture the earliest indicator of mortality [35]. However, SOFA score showed a negative correlation with the mortality, potentially because the poor clinical condition on day 1 makes those scores less useful for determining mortality. Follow-up ICU scores after the 1st post-transplant day may improve the predictive power of these tests and should include more patients.
The prognostic model of mortality in this study included prolonged mechanical ventilation during the ICU period after LT, which is a known risk factor for mortality after LT [36]. Intraoperative variables such as blood loss, urine volume, and postoperative renal failure may contribute to prolonged mechanical ventilation [37]. We developed a post-MELD for testing mortality at 1 year, and that included bilirubin and creatinine to measure early renal or allograft failure [32]. The MELD scores after the 5th post-transplant day showed the best mortality prediction, which was much earlier than 14th day post-MELD scores used previously [13]. Furthermore, we measured changes in MELD score over time. A sluggish decline in MELD score indicates slow recovery or a failing graft. After adjustment for other confounding factors, the sequential observation of MELD scores had better prognostic ability than static assessment on a designated post-transplant day. In particular, a decline <5 points (from the 1st day post-MELD score) until the 5th post-transplant day is the earliest predictor of mortality after LT.
This study has some inherent limitations as a retrospective study. Incomplete data was substituted with medians, but the patient population was small. A differentiated approach according to LT diagnosis (e.g. cirrhosis versus cancer) or cause of death might improve clinical application of the results. However, the use of 1-year mortality rates and changing post-MELD scores over time are important contribution of the study.
In conclusion, the post-MELD score could reflect 1-year mortality after LT, whereas pre-MELD, APACHE II, and SOFA scores did not. In particular, a sluggish decline in the post-MELD score of less than 5 points until the 5th day (from the 1st day post-MELD score) is the earliest independent predictor of 1-year mortality after LT.
Acknowledgements
We sincerely appreciate the support from the Clinical Research Coordinating Center (CRCC) at the College of Medicine, Catholic University of Korea for statistic analyses and the Transplantation Center at Seoul St. Mary's hospital for data collection on donor and recipient survival.