Effects of anesthesia on fluid volume kinetics after infusion of colloid solution during blood donation
Article information
Abstract
Background
The fluid kinetics of intravenously infused colloid during inhalation anesthesia and hemorrhage have not been investigated. We therefore assessed fluid space changes during infusion of hydroxyethyl starch solution after hemorrhage in conscious and desflurane-anesthetized individuals.
Methods
Following the donation of 400 ml of blood, 500 ml of hydroxyethyl starch solution was infused over 20 minutes into wakeful and desflurane-anesthetized volunteers. Blood was repeatedly sampled to measure hemoglobin concentration, a marker of plasma dilution, and fluid kinetic analysis was performed to evaluate changes in fluid space.
Results
Using a fluid kinetic model, we found that the mean volume of fluid space was 7,724 ± 1,788 ml in wakeful volunteers and 6,818 ± 4,221 ml in anesthetized volunteers, and the elimination rate constants were 7.1 ± 3.5 ml/min and 19.4 ± 4.6 ml/min, respectively.
Conclusions
Infusion of colloid after mild hemorrhage resulted in similar expansions of plasma volume in desflurane-anesthetized and conscious individuals. During anesthesia, however, the expansion of plasma volume by colloid was decreased and of shorter duration than observed in conscious patients.
Introduction
During surgery, appropriate administration of intraoperative fluids is important for maintaining cardiac output and tissue perfusion under conditions of ongoing fluid and electrolyte loss. Intravenously administered fluids may be crystalloid or colloid solutions, and the ability of a given fluid to expand intravascular volume can be influenced by many factors, including the type of infused fluid, the chosen form of anesthesia, and the volume status of the patient [1-3]. In a normovolemic state, anesthetics can alter the fluid kinetics of a crystalloid solution, by increasing the volume of distribution and decreasing the rate of excretion. These changes may promote intravascular fluid retention and peripheral fluid accumulation [3,4]. To compensate for lost blood volume after hemorrhage, extravascular fluid is mobilized into the vascular tree by a spontaneous process termed "transcapillary refill", and urinary output is suppressed [5]. Anesthetics per se inhibit transcapillary refill and delay urinary excretion [3,6,7]. Hemorrhage can also lead to intravascular retention of fluids, primarily caused by a reduction in the elimination rate constant [4,8,9]. Because anesthesia impairs transcapillary refill after hemorrhage [6], infusion of crystalloid solution to correct hypovolemia can lead to a higher level of extravascular water retention during anesthesia than is seen in wakeful subjects. Under conditions of combined hemorrhage and anesthesia, however, less tissue edema has been observed following infusion of colloid compared with crystalloid solution [10]. This is primarily attributable to a greater retention of colloid solution in the intravascular space, and a decrease in the volume of colloid solution exiting to the interstitial fluid.
To quantify the effects of desflurane anesthesia on the kinetics of infused colloid, we analyzed and compared the distribution and elimination of 6% (w/v) hydroxyethyl starch, dissolved in a balanced electrolyte solution (Hextend®, BioTime Inc, Berkeley, CA), in anesthetized and wakeful mildly hypovolemic volunteers using a fluid kinetic model [11]. Employing a computerized simulation procedure and with input of parameters obtained by kinetic analysis, we also calculated the optimal rates of infusion required to yield predetermined dilution levels in anesthetized and wakeful volunteers.
Materials and Methods
Sixteen healthy volunteers were recruited and divided into two groups of 8 subjects, a wakeful blood donor group (wakeful group, n = 8) and an anesthetized bone marrow (BM) donation group (anesthetized group, n = 8). The health status of each donor was assessed by history-taking, physical examination, and laboratory tests. All participants fasted overnight. The study protocol was approved by the Review Board of our institution, and all volunteers provided written informed consent.
Upon arrival in the blood donation room, both antecubital veins of individuals in the wakeful group were catheterized for blood donation and fluid infusion, followed by radial artery catheterization for blood sampling and continuous blood pressure monitoring. After completing donation of 1 unit (about 400 ml) of blood, each volunteer was intravenously infused with 500 ml of Hextend®, at a constant rate, over 20 minutes, via an infusion pump.
Individuals in the anesthetized group were denied fluids until 7 AM of the study day, at which time they were given Hartmann solution at a rate of 100 ml/h, followed by induction of anesthesia at 8 AM. The total volume of Hartmann solution infused until the end of induction was less than 200 ml, and fluid infusion was stopped when BM donation was completed. Anesthesia was induced with a bolus injection of thiopental sodium 5 mg/kg, rocuronium 0.5 mg/kg, and maintained with 6% desflurane-inspired concentration, N2O at 1 L/min, and O2 at 1 L/min. As in the wakeful group, antecubital veins and radial arteries were catheterized for fluid infusion, blood pressure monitoring, and blood sampling. When under anesthesia, each individual in the anesthetized group donated 1,200 ml of BM, with 800 ml of autologous blood being administered during the procedure. After completion of BM donation, 500 ml of Hextend® was infused, as in the wakeful group.
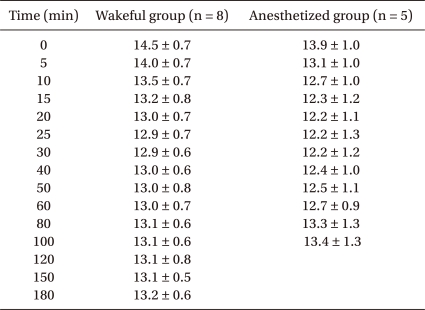
To calculate the changes in blood volume, we used hemoglobin (Hb) dilution method. The Hb concentration was measured using 1 ml arterial samples. At the time of each blood sampling, an initial volume of 2 ml of arterial blood was withdrawn for avoiding any contamination of the sample with other fluids. After a blood sample was drawn, the catheter was then flushed with 1-2 ml of heparinized saline and the withdrawn blood was discarded. In wakeful group, blood sampling was done before fluid infusion and at 0, 5, 10, 15, 20, 25, 30, 40, 50, 60, 80, 100, 120, 150 and 180 minutes after infusion started. Sampling was performed in anesthetized group before fluid infusion and at 0, 5, 10, 15, 20, 25, 30, 40, 50, 60, 80, 100 minutes after infusion started.
The distribution of the infused colloid was analyzed separately for the awake and anesthetized group using previously published one-compartment volume kinetic model [11-13], which can be summarized as follows: the infused fluid volume at a rate ki is distributed in an expandable body fluid space (v), which the fluid space strives to maintain at a target (baseline) volume (V). Fluid leaves the space at a fixed basal rate (kb), which represents baseline urinary excretion and evaporation to be 0.4 ml/min [14] and at a controlled rate proportional with a constant (kr) to the deviation from the target volume, V.
The dilution of plasma determined from blood samples was assumed to reflect an increase in the volume of expandable body fluid spaces. As plasma volume is a component of V, the dilution of plasma at time t was used to calculate (v(t) - V) / V using the formula:
(v(t) - V) / V= [(Hb0/Hbt) - 1] / (1-baseline hematocrit)
Baseline blood volume before fluid infusion (BV0) and blood volume (BVt) at any time (t) during fluid infusion were calculated as follows [13]:
BV0 (L) = 0.03219 × body weight (kg) + 0.3669 × height3 (m) + 0.6041 (for males), or
BV0 (L) = 0.033308 × body weight (kg) + 0.3561 × height3 (m) + 0.1833 (for females).
Baseline plasma volume was calculated by multiplying the baseline blood volume by (1 - hematocrit):
BVt = BV0 × hemoglobin0/hemoglobint
The following equation describes the volume change in v:
dv/dt = ki - kb - kr (v-V/V).
The unknown parameters v, V and kr were calculated by nonlinear least-squares regression using Matlab Version 6.8 (Math Works Inc., Natick, MA, USA). This program was also used in simulations and to create nomograms to identify combinations of infusion rates and infusion times producing a predetermined dilution of V [15]. The mean values of parameters obtained in the one-volume fluid space model were used to determine fluid regimens resulting in dilutions of 2%, 5%, 10%, 15%, 20%, and 25%. Simulations were also utilized to determine infusion rates that would maintain a steady-state dilution after target dilution had been attained.
All data are presented as means ± standard deviations (SD). Intergroup comparisons were performed using the Mann-Whitney test or the Wilcoxon Signed Rank test, and intra-group time-related variations were analyzed by repeated-measures analysis of variance. P < 0.05 was considered statistically significant.
Results
The withdrawal of 400 ml blood (inducing mild hypovolemia) was generally well tolerated [14]. There were no major adverse events and hemodynamic measurements remained constant throughout the study period in both groups. Rapid infusion of fluid produced no symptoms.
Three volunteers in the anesthetized group were excluded from final analysis because their volume kinetics could not be analyzed. Thus, eight wakeful and five anesthetized volunteers were included in analysis. There were no statistically significant intergroup differences in age, body weight, height, or baseline Hb concentration (Table 1). In both groups, Hb concentration decreased significantly during fluid infusion, with the maximal dilution of Hb observed 5 minutes after completion of fluid infusion, or 25 minutes after commencement of fluid infusion, increasing gradually thereafter (Table 2).
The target (baseline) volumes (the V values), as determined by volume kinetic analysis, were similar in the wakeful (7,724 ± 1,788 ml) and anesthetized (6,818 ± 4,221 ml) groups. In contrast, the elimination rate constant (kr) was significantly higher in the anesthetized (19.4 ± 4.6 ml/min) than in the wakeful (7.1 ± 3.5 ml/min) group (P < 0.01) (Fig. 1).

Fluid kinetic parameters in wakeful and anesthetized Volunteers. Data are median and interqurtile ranges. V: target body fluid space volume, Kr: elimination rate constant according to volume expansion ratio, ECF: extracellular fluid (= 200 ml/kg).
Peak blood volume expansion (BVt - BV0 / BV0) in both groups occurred 10 minutes after completion of fluid infusion (at 30 minutes), being 12.3 ± 3.4% in the wakeful and 14.6 ± 9.7% in the anesthetized group. In the wakeful group, this increase was maintained at both 60 minutes (11.3 ± 2.8%) and 180 minutes (9.6 ± 3.3%). In the anesthetized group, however, blood volume decreased rapidly, by 9.1 ± 4.7% at 60 minutes and 4.1 ± 3.6% at 100 minutes. After completion of fluid infusion, the blood volume expansion ratio was somewhat higher in the anesthetized than in the wakeful group, but the difference was not statistically significant (Fig. 2).
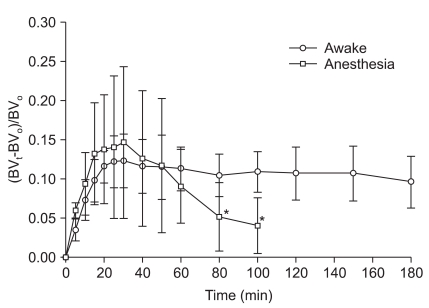
Changes in blood volume expansion during and after intravenous infusion of 500 ml of Hextend over 20 min after hemorrhage of 400 ml in wakeful (○) and desflurane-anesthetized (□) volunteers. Data are means and standard deviations. BVt: blood volume at any time during fluid infusion; BV0: Baseline blood volume before fluid infusion. *P < 0.05 compared with the wakeful group.
During fluid infusion after blood donation, the volume expansion ratio (v-V/V) of the fluid space increased in both groups, reaching a maximum 5 minutes after completion of infusion and decreasing gradually thereafter. At the time of maximal increase, the volume expansion ratio was significantly higher in the anesthetized (10.4 ± 2.6%) than in the wakeful (6.6 ± 1.6%) group (P < 0.05). However, the ratios were similar 100 minutes after completion of infusion (5.5 ± 1.4% vs. 5.6 ± 1.2%, respectively) (Fig. 3).

Fluid kinetic model-predicted dilution-time curve of the primary fluid space during and after intravenous infusion of 500 ml of Hextend over 20 min after hemorrhage of 400 ml in wakeful (○) and desflurane-anesthetized (•) volunteers. v: expandable body fluid space, V: baseline body fluid volume. *P < 0.05 compared with the wakeful group.
Infusion strategy nomograms, showing the relationship between infusion rate and infusion time of various fluid regimens to replace blood lost with Hextend® in both wakeful and anesthetized subjects, were created by computer simulation using one-compartment volume model kinetic parameters (Fig. 4). These nomograms showed the amount of fluid required to reach a predetermined dilution (and/or to restore normovolemia) by combining various infusion rates and infusion times.
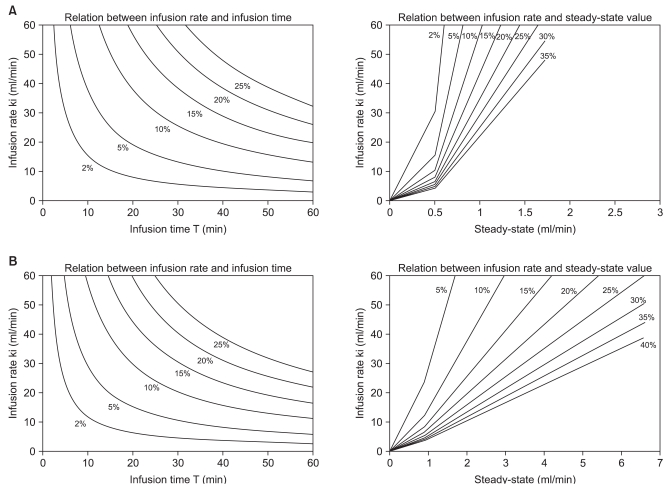
Infusion strategy nomogram showing (left) the relationship between infusion rate and infusion time and (right) the infusion rate required to maintain a steady-state dilution, estimated from both infusion rate and infusion time during the first phase, with reference to Hextend infusion after mild hypovolemia (400 ml) in wakeful (A) and anesthetized (B) individuals.
Discussion
We show here that anesthesia in mildly hypovolemic patients increased kr values and rapidly decreased the effect of Hextend® on blood volume expansion over time.
If the body fluid space is considered to be a space surrounded by an elastic wall, like a balloon filled with water, and if fluid shifting occurs according to a one- or two-volume space model, then infusion of a crystalloid solution can be interpreted using either space model, whereas infusion of a colloid solution can be interpreted only by employing a one-volume space model [11,13,16]. We therefore utilized a one-volume space model in the present study.
Following hemorrhage, a considerable volume of extravascular fluid flows into the intravascular space, powered by a physiological compensatory mechanism termed "transcapillary refill" or "autotransfusion", to compensate for the loss of volume [17]. Factors contributing to transcapillary refill include a decrease in capillary hydrostatic pressure caused by the constriction of arterioli [18], an increase in total intravascular protein [19], a rise in lymphatic flow [17], and movement of interstitial fluid into the intravascular space caused by the osmotic effect of hyperglycemia [20]. Transcapillary refill varies depending on the volume hemorrhaged, the time since hemorrhage, and the use of anesthetics [6,21]. Isoflurane anesthesia has been reported to inhibit compensatory transcapillary refilling after hemorrhage, resulting in decreased expansion of intravascular volume compared with what is seen in wakeful subjects [6]. Following normotensive (15%) hemorrhage, intravascular volume restoration in anesthetized individuals was only 23% that of wakeful individuals. Moreover, the rate of transcapillary refill varied from 0 to 11.2 ml/min, depending on the time after hemorrhage and any use of an anesthetic agent, but was most rapid during the first 40 minutes after hemorrhage. In addition, although studies have shown that approximately 40% of bled volume was restored at a rate of 10.5 ml/min during the first 40 minutes after hypotensive (45%) hemorrhage, other reports have described much lower volumes and rates. All these factors including physiologic compensation mechanism, the volume hemorrhaged, and anesthesia might affect the kinetics of fluid infusion and the volume requirement after hemorrhage [5].
In the anesthetized state, increased concentrations of renin and aldosterone, systemic hypotension, and renal vasoconstriction have been found to result in a lower glomerular filtration rate (30-50% that observed in wakeful individuals), a drop in renal blood flow (by 40-60%), and a fall in urinary flow rate (by 34%), all of which can increase retention of infused fluid [3]. The volume expansion effect of fluid infused after hemorrhage, in both anesthetized and wakeful individuals, may be affected by differences in these factors. The elimination rate constant (kr), representing the clearance of infused fluid from plasma, remained constant in individuals with normal kidney function, unless the amount of infused fluid was extremely large (50 ml/kg) [22]. Moreover, kr was highly correlated with urinary output. However, surgical stress, anesthetics, and hemorrhage, can all lead to changes in kr [3-5]. We found that kr was larger in the anesthetized than in the wakeful group, a finding in conflict with previous reports, which showed that crystalloid infusion after hemorrhage [5] and under anesthesia [4] decreased both urinary output and kr. However, kr values in anesthetized individuals may represent total elimination from plasma, including both urinary excretion and peripheral accumulation, rather than urinary excretion alone [23]. Our results therefore suggest that, following mild hemorrhage and the infusion of Hextend®, fluid elimination from plasma to the extravascular space may be greater in anesthetized than in wakeful patients. However, our results cannot be directly compared with those of other studies, as we did not measure urinary output. Moreover, our results suggest that the volume expansion effect of colloid solution is not sustained during anesthesia, resulting in greater water retention in the extravascular space in anesthetized than in wakeful individuals.
Although the difference was not statistically significant, we found that V values with use of Hextend® infusion were smaller in anesthetized than in wakeful individuals under conditions of mild hypovolemia, and this may reflect extravascular accumulation, even during fluid infusion. The volume expansion ratio of the fluid space was also higher in the anesthetized than in the wakeful group. This may be associated with the larger kr value observed for anesthetized subjects. If, however, the target volume of anesthetized patients was smaller because of individual variation, the expansion ratio would be greater in this group. These results are consistent with those reported previously [23].
Computer simulations using one-compartment volume kinetic parameters for wakeful and anesthetized individuals (Fig. 3) can be used as nomograms to determine infusion rates and infusion times required to produce a desired dilution of plasma, and to maintain a steady-state dilution after mild blood loss (400 ml). For example, a plasma dilution of 5% (v/v) in wakeful individuals requires an infusion rate of 20 ml/min for 19 min or 40 ml/min over 9.5 min (total, 380 ml). A subsequent infusion rate of 0.55 ml/min or 0.7 ml/min, respectively, of additional fluid, would be required to maintain a steady-state plasma dilution of 5% (v/v). In anesthetized individuals, however, an infusion rate of 20 ml/min over 15 min or 40 ml/min over 7.5 min (total, 300 ml) would be required to attain a plasma dilution of 5% (v/v), and an infusion rate of 0.8 ml/min or 1.2 ml/min, respectively, of additional fluid would be required to maintain this dilution. Thus, compared with wakeful individuals, anesthetized patients require smaller volumes of fluid to achieve a given plasma dilution, but higher rates of additional fluid infusion to maintain a steady state. In addition, our nomograms (Fig. 3) are valid for colloid fluid replacement after mild blood loss (400 ml) only in wakeful and anesthetized healthy individuals. Thus, caution should be exercised when applying our nomograms to other conditions, such as severe blood loss, in which situations the volume kinetics might be different.
This study had several limitations. First, precise comparison between the two groups was difficult because autologous blood was transfused during BM donation, before the infusion of colloid, in anesthetized individuals, although the total volume of blood loss was the same in both wakeful and anesthetized individuals. This may have affected the volume kinetics of the infused solution. Secondly, because hypotension per se may affect water retention and fluid kinetics [7], different results may have been obtained following the loss of large amounts of blood, leading to hypotension. Hence, our findings in individuals with mild hypovolemia should not be extrapolated to those with moderate or massive hemorrhage.
In conclusion, we utilized volume kinetic analysis to compare the effects of colloid infusion on volume expansion in mildly hypovolemic anesthetized and wakeful individuals. We found that such infusion had similar effects of volume expansion in the two groups, but the volume expansion effect rapidly decreased in the anesthetized group. Because the elimination rate constant was significantly larger in the anesthetized than in the wakeful group, leading to increased extravascular water retention in the former subjects, care should be taking when infusing colloids during anesthesia. The nomograms created from parameters of our fluid kinetic model may be useful in guiding fluid infusion after mild hemorrhage.
Notes
The results from this study have been presented at the 85th Annual Scientific Meeting of The Korean Society of Anesthesiologists, November 6, 2008.