Maintenance of nitric oxide inhalation to a patient with hemoperitonium and acute respiratory distress syndrome during anesthesia -A case report-
Article information
Abstract
Inhaled nitric oxide (NO) is occasionally used to treat hypoxemia for patients with acute respiratory distress syndrome (ARDS) in the intensive care unit (ICU). However, it is controversial whether or not to maintain inhalation of NO during general anesthesia because of complications, such as nitrogen dioxide (NO2) production, methemoglobinemia, and inhibition of platelet aggregation. In this case, a 67-year-old male fell from a roof and was brought to an emergency care center. During management, he vomited gastric contents and aspirated. In spite of tracheal intubation and mechanical ventilation with high oxygen therapy, the hypoxia did not improve. NO inhalation with mechanical ventilation was performed to treat hypoxemia due to ARDS in the ICU. We maintained the NO inhalation during the surgery for a hemoperitonium. The surgery was completed without intra-operative hemodynamic instability or any complications.
Introduction
One of the toxic pollutants, NO, is known as a free radical gas. In recent years, however, NO is an endothelial-derived vasodilator molecule and has been examined as a supplemental treatment agent for pulmonary hypertension. It has also been reported that the peri-operative portopulmory hypertension which occurs during liver transplantation is controlled by the administration of NO and prostacyclin [1,2]. In patients with ARDS, ongoing studies are determining whether or not oxygenation can be enhanced by the pulmonary vasodilation as a supplemental treatment agent [3]. Because the side effects due to NO2 occurring during the persistent use of NO cannot be overlooked, that the safety of continued use of NO during general anesthesia when used pre-operatively needs to be determined.
We managed a case of ARDS due to pulmonary aspiration in which NO which was used in the ICU to improve oxygenation and was maintained during abdominal surgery. Herein we report our case with a review of the literature.
Case Report
A 67-year-old man weighing 80 kg had no other notable findings than a history of diabetes mellitus, and the patient visited the emergency care center with a chief complaint of hemoperitonium occurring after a fall injury from a 3 m height. At the time of admission, the patient had a clear mentality and spontaneous respirations. Due to persistent intra-abdominal bleeding, the patient had the following findings: blood pressure (BP), 85/50 mmHg; and heart rate (HR), 110 beats/min. During treatment at the emergency care center, the patient aspirated gastric contents, which led to tracheal intubation. Despite the infusion of dopamine (20 µg/kg/min), the patient remained hypotensive. To stop the abdominal bleeding, emergency surgery was performed. Sedation was continued post-operatively, and the patient was transferred to the ICU intubated. On postoperative day 1, during treatment with sedation and ventilator care in the ICU, hemodialysis was performed due to the occurrence of sepsis and acute renal failure (ARF). This was followed by the occurrence of ARDS due to pulmonary aspiration (Fig. 1). Controlled mechanical ventilation (CMV) was initiated with the following settings: tidal volume (TV), 480 ml; respiratory rate (RR), 20 breaths/min; FiO2, 1.0; and positive end expiratory pressure (PEEP), 15 mmHg. An arterial blood gas analysis (ABGA) was performed which showed the following: pH, 7.01; PaCO2, 60 mmHg; PaO2, 47 mmHg; HCO3-, 15.1 mmol/L; and SaO2, 58%. To improve oxygenation, NO was administered at a dose of NO from 3:00 pm on post-operative day 1 (Table 1).
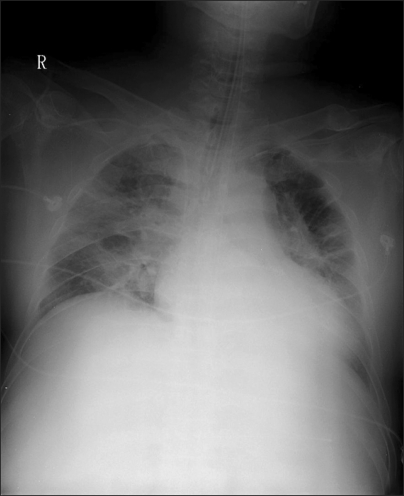
Chest AP before using nitric oxide (NO) treatment shows increased perihillar opacity, pulmonary edema in lung fields.
Until post-operative day 2 following the use of NO, an ABGA was performed under the following CMV settings: TV, 450 ml; RR, 22 breaths/min; FiO2, 1.0; and PEEP, 20 mmHg. The ABGA showed as the following: pH, 7.27; PaCO2, 46.1 mmHg; PaO2, 126.7 mmHg; HCO3-, 20.7 mmol/L; and SaO2, 98.4%. Thus, oxygenation was gradually improving (Table 1). Due to the persistent presence of abdominal bleeding, an emergency repeat surgery was performed. While maintaining NO inhalation, the patient was transferred to the operating room (Fig. 2).
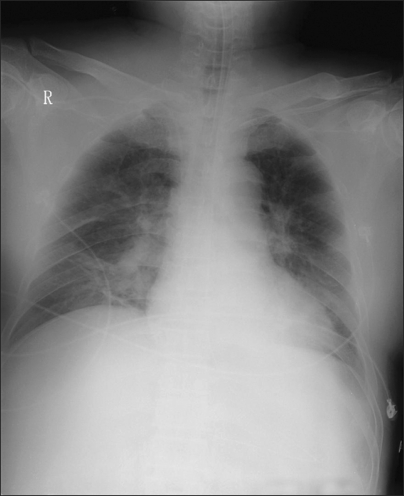
Chest AP after using nitric oxide treatment shows more resolved pulmonary edema, but still remained prominent vascular marking and consolidation on the right lower lobe.
Upon arrival in the operating room, the patient had the following implants: a catheter in the right subclavian vein for central venous pressure (CVP) monitoring, a catheter in the right femoral vein for fluid supply, a catheter in the right radial artery for a continuous arterial blood pressure monitoring, and a colostomy. To maintain vital signs, the patient received an infusion of vasopressin (1 unit/hr), dopamine (15 µg/kg/min), norepinephrine (0.8 µg/kg/min), and bicarbonate (20 mEq/hr). The patient also had a BP of 103/55 mmHg, a HR of 110 beats/min, and a CVP of 21 mmHg. Mechanical ventilation was performed using a ventilator (Savina, Drager, Germany) which was used in the ICU. With continued CMV (TV, 400 ml; RR, 22 breaths/min; FiO2, 1.0; and PEEP, 18 mmHg), the measurements were as follows SaO2, 85-90%; and a peak inspiratory pressure (PIP), 34 mmHg. NO was infused to the area which was 30 cm remote from the Y-piece of the inspiratory limb of the ventilator using a NO delivery system (HY-1002; Metran Co., Germany). In the connecting part between the Y-piece and inspiratory limb, using an expiratory gas analyzer (PrinterNOx; Micro Medical Ltd., England), the concentrations of NO and NO2 were measured (Fig. 3 and 4).

The photograph shows a NO gas dosage machine (PrinterNOx, Micro Medical Ltd., England), NO gas supplying line (white arrow) and monitoring line (black arrow).

The photograph shows a NO gas supplying line (white arrow) that is connected as 30 cm from the Y-piece on the inspiratory limb and the monitoring line (black arrow) is placed at the inspiratory limb just behind the Y-piece.
The patient's mental status was sedated with midazolam, which was used in the ICU (the bispectral index [BIS] was 8). Because the ventilator which was used in the ICU was also used in the operating room, due to a lack of availability of a vaporizer, the induction and maintenance of anesthesia were performed using total intravenous anesthesia. With reference to BIS measurements, a small dose of propofol (2.0 µg/ml) and remifentanil (2.0 ng/ml) were administered using a target-controlled infusion (Orchestra™; Fresenius Vial, France). A muscle relaxant, atracurium, was intravenously adminsitered at a dose of 20 mg/hr. Intra-operatively, BIS was abruptly increased from approximately 10 to 35. Because the BP was not sufficiently higher for the enhancement of the propofol infusion, to maintain the depth of anesthesia, midazolam was intravenously injected twice at a dose of 1 mg. Intra-operatively, the concentration of NO was maintained at 5.0-9.5 ppm, and NO2 was 0-0.8 ppm. With aggravation of the patient's vital signs, a Swan-Ganz catheter for pulmonary artery pressure (PAP) monitoring was not placed. Following the initiation of surgery, abdominal decompression was performed. Meanwhile, the BP decreased from 120/50 mmHg to 95/40 mmHg. The CVP decreased from 21 mmHg to 15 mmHg. The SaO2 increased from 90% to 100%. The PIP decreased from 34 mmHg to 24 mmHg. Blood was used as fluid therapy and an intravenous infusion of phenylephrine resulted in an elevation of BP to approximately 110/50 mmHg. Thereafter, during the surgery, there were no notable changes in the patient's status. At the time the surgical procedure was completed, with a continuous infusion of vasopressin (1 unit/hr), dopamine (15 µg/kg/min), norepinephrine (0.8 µg/kg/min) and bicarbonate (20 mEq/hr), the BP was 123/56 mmHg, the HR was 78 beats/min, and the CVP was 13 mmHg. Mechanical ventilation was finally set at a TV of 500 ml, a RR of 16 breaths/min, a FiO2 of 0.8 (O2, 3.2 L/min; and N2O, 0.8 L/min), and a PEEP of 10 mmHg in an effort to not further increase the PIP, TV, and minute volume. With the maintenance of the concentration of NO at 5.0-10 ppm, an ABGA was performed, and showed the following: pH, 7.18; PaCO2, 45 mmHg; PaO2, 430 mmHg; HCO3-, 16.8 mmol/L; SaO2, 100%; and PIP, 25 mmHg (Table 1). At this time, the BIS was 13-20. The intra-operative blood loss was 1,000 ml. With the transfusion of PRC (4 units), FFP (6 units), and PC (10 units), the Hb was maintained at 9.2 mg/dl. The anesthetic and surgical time was 2 hours and 50 minutes and 2 hours and 20 minutes, respectively.
Following surgery, the patient was transferred to the ICU. The CMV settings were as follows: TV, 550 ml; RR, 16 breaths/min; FiO2, 0.8; and PEEP, 10 mmHg. An ABGA analysis showed as the following: pH, 7.20; PaCO2, 39 mmHg; PaO2, 305 mmHg; HCO3-, 15.2 mmol/L; and SaO2, 100% (Table 1). The patient's status was considered to be improved. The use of NO was therefore discontinued (Fig. 5). Following the repeat surgery, however, the abdominal bleeding persisted. There were no further improvements in ARDS. On post-operative day 1, at approximately 5:00 pm, with the following CMV settings: TV, 500 ml; RR, 20 breaths/min; FiO2, 1.0; and PEEP, 20 mmHg, an ABGA was obtained (pH, 7.29; PaCO2, 42 mmHg; PaO2, 71 mmHg; HCO3-, 20.2 mmol/L; and SaO2, 92%; Table 1). Due to a lack of response to an increased dose of epinephrine and norepinephrine, systolic blood pressure was not maintained at >80 mmHg. At dawn on post-operative day 2, due to multiple organ failure, including ARF, sepsis, aspiration pneumonia, and respiratory failure, the patient expired.
Discussion
In patients who concurrently have hypoxic pulmonary vasoconstriction (HPV) and pulmonary hypertension due to alveolar hypoxia, such as ARDS, NO has been used as a supplemental treatment agent when hypoxemia is not resolved with oxygen supply and the appropriate mechanical ventilation. The current patient developed ARDS due to pulmonary aspiration. Due to persistent intra-abdominal bleeding, the abdominal pressure was elevated, and the patient had decreased pulmonary function. The most severe form of acute lung injury (ALI), ARDS, has been reported to have various causes. It can be defined as cases in which the PaO2/FiO2 is <200 mmHg (ALI PaO2/FiO2 <300 mmHg) and a pulmonary-capillary wedge pressure is <18 mmHg, despite the presence of bilateral pulmonary infiltrates on chest radiography [4]. According to ARDS NET reports [5], to reduce pulmonary injury due to mechanical ventilation and to assure that the PIP and plateau pressure does not exceed 30 cmH2O, a low TV (4-8 ml/kg), and a respiratory rate which is appropriate for each age group, has been recommended. The same methods are also applied to mechanical ventilation during anesthesia. In cases in which sufficient oxygenation cannot be achieved by these methods, although PEEP which is used as a supplemental method should prevent the alveolar atelectasis and reduce an intrapulmonary shunting, the effects of NO in reducing the intrapulmonary shunt due to pulmonary reperfusion from the affected sites to normal alveoli has been improved. Besides, there are also effects of NO in lowering the pulmonary hypertension [6,7]. But it cannot enhance the survival of patients. Also, due to side effects occurring following a long-term use of NO, it is not generally used for cases of ALI or ARDS. In patients who have severe hypoxia or life-threatening pulmonary hypertension, it is used as a supplemental treatment agent during a short-term period [7].
The patient reported herein had severe ARDS because of the elevation of abdominal pressure due to the persistent intra-abdominal bleeding, even after surgery, as well as pulmonary aspiration occurring at the emergency care center at the time of admission. With a single use of positive pressure ventilation, the hypoxia was not improved in the ICU. As a supplemental treatment agent, NO was used at a dose of 5.0-9.5 ppm. As a result, the ABGA findings were improved from a PaO2 of 47 mmHg and an oxygen saturation 58% prior to the use of NO at a FiO2 of 1.0 to a PaO2 of 126.7 mmHg and a SaO2 of 98.4% following the use of NO.
NO has been used to improve pulmonary oxygenation. But the long-term use of NO may cause several complications, and these include the synthesis of methemoglobin, the synthesis of nitrogen oxide, and an inhibitory effect on platelet aggregation. In the current case, however, the reasons that NO was maintained during surgery are that discontinuation of NO would aggravate the pulmonary hypertension, increase the pulmonary vascular resistance, decrease the cardiac output, decrease the mixed venous oxygen saturation (SvO2), decrease the systemic arteral blood pressure, and decrease the PaO2. Christenson et al. [8] conducted a study in 31 patients with acute hypoxemic respiratory failure (AHRF) who inhaled NO. According to this study, following the discontinuation of NO treatment, such parameters as PaO2, SvO2, and PAO2/FiO2 were significantly decreased. Kim et al. [9] reported that rebound pulmonary hypertension developed frequently in cases in which the use of NO was abruptly discontinued at a lower dose after its concentration was lowered during a short-term period. Rebound pulmonary hypertension occurs due to various causes and obscure mechanisms. These authors therefore proposed that a gradual discontinuation be done through repeated attempts during a long-term period with special attention paid to hemodynamic stability.
In regard to the optimal NO concentration, there are various reports [7,10-12]. Continual use of NO (10 ppm) caused a left shift of the dose-response curve and this leads to an improvement of arterial oxygenation and mean PAP. But a maximal dose of NO (100 ppm) may aggravate oxygenation [7]. In association with this, Adhikari et al. [13] conducted a systematic review and a meta-analysis and these authors reported that methemoglobinemia and ARF would occur in cases in which a high dose of NO (>80 ppm) was used for several days.
Nishimura et al. [10] maintained that NO2 was not generated in cases in which NO would be used at a dose <20 ppm. According to another study, in cases in which NO was used at a dose of 20 ppm at FiO2 0.9, NO2 was generated at a dose of < approximately 0.4 ppm [12]. Based on these reports, in cases in which the concentration of NO was maintained as <20 ppm during anesthesia, the concentration of NO2 would not exceed 5 ppm as a safety limit of NO2 [14]. Accordingly in the current case, a low dose of NO (5.0-9.5 ppm) was used during anesthesia.
In the current case, NO was used since one day prior to surgery. Therefore, the period of use was not relatively longer and a low dose of NO was used. Complications due to the use of NO did not occur prior to and following the surgery. The surgery went well without specific problems. It is regrettable, however, that changes in the PAP pre-operatively and post-operatively could not be measured due to a lack of the preoperative preparation of a Swan-Ganz catheter for a PAP monitoring.
If the occurrence of NO2 is minimized, the problems occurring following a sudden discontinuation of NO use pre-operatively rather than complications occurring because of the use of NO would be more problematic. In conclusion, the maintenance of NO inhalation during anesthesia, rather than a sudden discontinuation of NO use pre-operatively, would be beneficial for the hemodynamic stability of patients.

