Accuracy of suprascapular notch cross-sectional area by MRI in the diagnosis of suprascapular nerve entrapment syndrome: a retrospective pilot study
Article information
Abstract
Background
Previous studies have demonstrated that morphological changes in the suprascapular notch are closely associated with suprascapular nerve entrapment syndrome (SNES). Thus, we hypothesized that the suprascapular notch cross-sectional area (SSNCSA) could be a good diagnostic parameter to assess SNES.
Methods
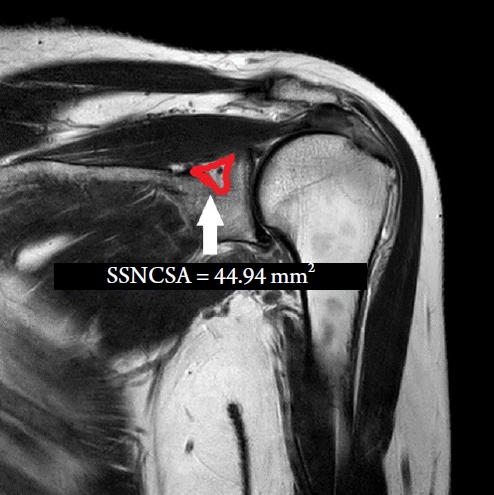
We acquired suprascapular notch data from 10 patients with SNES and 10 healthy individuals who had undergone shoulder magnetic resonance imaging (S-MRI) and had no evidence of SNES. T2-weighted coronal magnetic resonance images were acquired from the shoulder. We analyzed the SSNCSA at the shoulder on S-MRI using our image-analysis program (INFINITT PACS). The SSNCSA was measured as the suprascapular notch, which was the most affected site in coronal S-MRI images.
Results
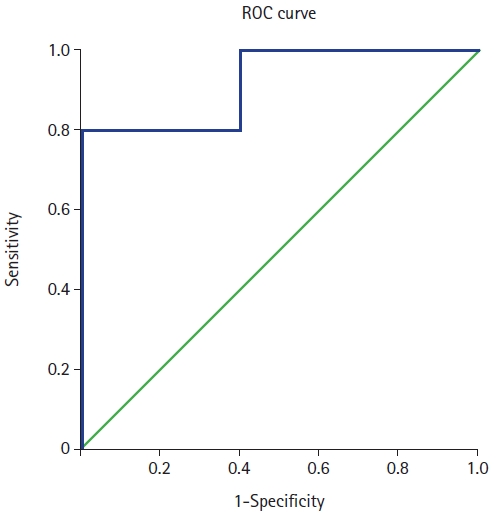
The mean SSNCSA was 64.50 ± 8.93 mm2 in the control group and 44.94 ± 10.40 mm2 in the SNES group. Patients with SNES had significantly lower SSNCSA (P < 0.01) than those in the control group. Receiver operating curve analysis showed that the best cut-off of the SSNCSA was 57.49 mm2, with 80.0% sensitivity, 80.0% specificity, and an area under the curve of 0.92 (95% CI [0.79, 1.00]).
Conclusions
The SSNCSA was found to have acceptable diagnostic properties for detecting SNES. We hope that these results will help diagnose SNES objectively.
Introduction
Suprascapular nerve entrapment syndrome (SNES) is a peripheral neuropathy caused by compression of the suprascapular nerve. SNES is uncommon in patients with shoulder dysfunction. However, SNES has clinical implications because it innervates approximately 60–70% of the shoulder joint and often leads to pain over the lateral and posterior aspects of the shoulder as well as weakness of the infraspinatus or supraspinatus muscles due to suprascapular nerve innervation [1–6]. Trauma or traction injury due to repetitive overhead activities or massive rotator cuff tears occurs in SNES [7–10]. SNES should be differentiated from disorders of the cervical part of the spinal cord, damage to the brachial plexus, cervical discopathy, or diseases of the shoulder joint, such as damage to the rotator cuff or degeneration of the shoulder [11–13]. Thus, an exact diagnosis is important for managing SNES.
The diagnosis of SNES is typically based on physical examinations and interview [14,15]. Other additional examinations for the diagnosis of SNES include imaging modalities (ultrasonography, X-ray, and computed tomography), electromyography (EMG), and assessment of conduction velocity from the neck nerve point to the supraspinatus muscles [10,16–19]. Although EMG is the gold standard for the diagnosis of SNES, shoulder magnetic resonance imaging (S-MRI) is also useful for the analysis of pathologic abnormalities of the suprascapular notch [2]. Podgórski et al. [20] reported that there are many anatomical variations in the suprascapular notch region, and the shapes of the suprascapular notch are highly diverse. In addition, the suprascapular nerve is most commonly compressed at the suprascapular notch [2]. However, few studies have investigated how morphological changes in the suprascapular notch affect the SNES. Moreover, no studies have examined the clinical optimal cut-off point of the suprascapular notch cross-sectional area (SSNCSA to diagnose SNES).
Therefore, to assess the relationship between SNES and the suprascapular notch, we developed a new morphological diagnostic parameter called SSNCSA. The SSNCSA has not yet been analyzed for its correlation with SNES. We hypothesized that the SSNCSA is an important morphological parameter in the diagnosis of SNES.
Materials and Methods
Participants
This original research protocol was approved by The Catholic Kwandong University Institutional Review Board (IRB no. IS21RISI0021). We reviewed electronic medical records of patients who had visited the shoulder orthopedic clinic with SNES from November 2015 to December 2020 and who had taken S-MRI within six months of the visit.
The SNES group included patients diagnosed with SNES by attending physicians according to their history, physical examination, and imaging modality. In addition, the final diagnosis was confirmed using EMG. Exclusion criteria were as follows: (1) history of scapular fracture, (2) history of shoulder surgery, and (3) no available S-MRI. Patients who underwent S-MRI and had no structural abnormalities were included in the control group.
The SNES group comprised 10 patients. There were 8 (80.0%) men and 2 (20.0%) women, with an average age of 43.90 ± 15.57 years (range, 18–60 years) (Table 1). To compare SSNCSA between individuals with and without SNES, we enrolled a control group consisting of individuals who wanted to undergo S-MRI for accurate diagnosis. The control group included patients with shoulder pain who wanted to undergo S-MRI. Moreover, patients in the control group did not show any abnormal findings on S-MRI. The control group comprised 10 individuals (6 men and 4 women) with a mean age of 42.70 ± 13.28 years (range, 20–73 years).
Imaging parameters
S-MRI was performed on a 3.0T MR unit (MAGNETOM Skyra; Siemens Medical Solutions, Germany) and 3T Ingina (Philips Medical Systems, The Netherlands) scanners, and T2-weighted coronal plane turbo spin-echo images were acquired from all enrolled patients. The following S-MRI sequences were used: slice plane axial, field of view of 160 × 160 cm, repetition time of 619.0 milliseconds, echo time 13.0 milliseconds, flip angle 35 degrees, slice thickness 3.00 mm, matrix size 512 × 307 pixels, number of signals averaged = 2, scan time 4 min 32 s, and 3 > echo train length.
Image analysis
SSNCSA measurements were done by a board-certified pain specialist with 15 years of experience who was blinded to the shoulder state. We obtained coronal T2-weighted turbo spin echoS-MRI images that presented the best visualization of the suprascapular nerve. We measured SSNCSA on S-MRI using our image-analysis program (INFINITT PACS, ver. 3.0; INFINITT Healthcare, Seoul, Korea) (Fig. 1).
Statistical analysis
We compared the SSNCSA between the SNES and normal individuals using independent t-tests. Statistical significance was set at P < 0.05. Receiver operating curve (ROC) curve analysis was used to present the diagnostic values of SSNCSA in the diagnosis of SNES, and the diagnostic values included the cut-off points, area under the curve (AUC), specificity, and sensitivity. SPSS (version 22.0; IBM Inc., USA) was used to analyze the collected data.
Results
Demographic characteristics were not significantly different between the groups. The average SSNCSA was 64.50 ± 8.93 mm2 in the control group and 44.94 ± 10.40 mm2 in the SNES group (Table 1). Patients with SNES had significantly lower SSNCSA scores (P < 0.01) than those in the control group (Table 1). The ROC analysis showed that the best cut-off value of the SSNCSA was 57.49 mm2, with 80.0% sensitivity, 80.0% specificity, and the AUC of SSNCSA was 0.92 (95% CI [0.79, 1.00]) (Table 2, Fig. 2).
Discussion
This pilot study aimed to determine the clinical implications of SSNCSA in SNES. The present study showed that SSNCSA of 57.49 mm2 had 80.0% sensitivity and 80.0% specificity for predicting SNES. This result demonstrates that the SSNCSA could be a meaningful predictor of SNES.
SNES is a neuropathic condition in which the suprascapular nerve is compressed along the pathway. Traumatic injuries, such as clavicular fractures, scapular fractures, proximal humerus fractures, and dislocation of the acromioclavicular joint or shoulder, are common causes of suprascapular nerve damage [4,12,13,21]. Most importantly, suprascapular nerve compression most commonly occurs at the suprascapular notch, and its symptoms and signs are caused by nerve compression based on morphological changes in the suprascapular notch [2,22,23]. Structures around the suprascapular nerve can be compressed and injured by various mechanical factors. Direct compression in the suprascapular notch region (e.g., labral cyst, ganglion cyst, tumor) [11], continuous nerve irritation after rotator cuff injuries, or inflammation of the shoulder can all lead to SNES [3,24].
SNES diagnosis is based on patient history and physical examinations while ruling out other similar pathologies, including cervical radiculopathy, cervical discopathy, and various rotator cuff injuries. Imaging studies can also be used for diagnosis [14,15]. Ultrasound, X-ray, computed tomography, and MRI provide information on the suprascapular nerve and surrounding structures, helping diagnose SNES [15,25,26]. Nerve conduction velocity and EMG are the gold standards for SNES diagnosis [14]. However, nerve conduction velocity and EMG cannot be performed in all patients and the efficacy of EMG is low. Although EMG can confirm nerve conduction problems or muscle weakness, it is not useful for the differential diagnosis of various shoulder diseases. Moreover, negative EMG results cannot rule out SNES when clinical signs and symptoms are highly suspicious of SNES [14]. Therefore, MRI is more commonly performed in shoulder injury patients than EMG, and the diagnostic criteria of MRI can be a useful tool to diagnose SNES.
Even though it has been reported that SNES is more likely to occur in patients with a V-shaped or narrow suprascapular notch, a significant correlation between the suprascapular notch type and SNES has not been confirmed. Ürgüden et al. [9] reported that Rangachery types 4 and 5 of the SSN may increase the risk of suprascapular nerve injury during rotator cuff tear operations. However, no clinical studies have been conducted to prove this theory. Polguj et al. [17] reported that the size of the suprascapular notch is a major risk factor for SNES [22]; however, there is no study to analyze suprascapular notch objectively. In other words, the narrowed suprascapular notch is considered a major morphological parameter of SNES. Therefore, we believe that analyzing the cross-sectional area of the suprascapular notch is the most important factor in the diagnosis of SNES, and we designed the present study to prove the correlation between SSNCSA and SNES.
As the bone margin of the suprascapular notch has a wavy or curved contour and multiple signal intensities within the narrowed site, it is difficult to measure. Thus, the length or thickness of the suprascapular notch is not an appropriate measurement for diagnosing SNES. Instead, the cross-sectional area of the suprascapular notch may predict SNES effectively because the SSNCSA, which measures the whole cross-sectional area of the suprascapular notch represents the space of the SSN limited by surrounding structures. This study demonstrated that SSNCSA is a good morphological measurement diagnostic tool for SNES.
This study has some limitations. First, SNES has multiple causes such as rotator cuff tears, trauma, and repetitive overhead activities. Additionally, the structures around the suprascapular nerve, such as the supraspinatus muscle, infraspinatus muscles, suprascapular ligament, suprascapular notch, and spinoglenoid notches, were not considered. However, we focused only on the suprascapular notch, where the suprascapular nerve is most commonly compressed. In future studies, we will investigate other anatomical structures that affect SNES, especially the spinoglenoid notch, where the suprascapular nerve is also commonly compressed. Second, there might be some errors in the measurements of SSNCSA on S-MRI. Although we attempted to analyze this morphologic measurement method in the best plane that presents the suprascapular notch in the coronal image section, the coronal images we measured in the section image could be inhomogeneous because of differences in the cutting level or angle in the S-MRI as a result of individual anatomical differences and technical errors. Third, there are several alternative imaging diagnostic tools to evaluate SNES, such as ultrasound examination or computed tomography; however, this study analyzed only the measurement of the SSNCSA on S-MRI. Fourth, functional instability was not analyzed because it is a subjective finding that may vary from one interpretation to another. The goal of this study was to provide objective morphological indicators. Fifth, only a small number of patients were enrolled in the study. We enrolled all patients diagnosed with SNES at our hospital; there were only 10 SNES patients. Although this pilot study investigated a small number of patients, it is valuable because it provides diagnostic criteria using S-MRI, especially SSNCSA. Sixth, since this was a retrospective study, patients who underwent S-MRI and had no structural abnormalities were enrolled in the control group. The control group might have experienced shoulder pain and may not represent the normal population. In a future study, healthy individuals should be recruited in the control group and scanned for S-MRI prospectively, and a more accurate SSNCSA of normal people can be obtained.
Despite several limitations, we present the diagnostic criteria for SNES using S-MRI for the first time, especially using SSNCSA. In addition, the present study showed that the SSNCSA can be an objective and useful diagnostic tool for SNES.
We concluded that these data strengthen the finding that SSNCSA plays a significant role in determining SNES.
Acknowledgements
The authors would like to thank SoYoon Park, Jae Ni Jang, Yun-Hong Kim, and Won-Jun Choi for managing the administrative activities related to the conduct of the study.
Notes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Jiyeon Park (Formal analysis; Investigation; Resources; Writing – review & editing)
Min-Ying Su (Writing – review & editing)
Young Uk Kim (Conceptualization; Data curation; Formal analysis; Investigation; Project administration; Supervision; Writing – original draft)