Prevention of epidural catheter migration: a comparative evaluation of two tunneling techniques
Article information
Abstract
Background
Epidural analgesia failure episodes can be reduced by catheter fixation techniques with a lower incidence of catheter migration. In this clinical study, we compared the roles of two epidural catheter tunneling techniques for the prevention of epidural catheter migration.
Methods
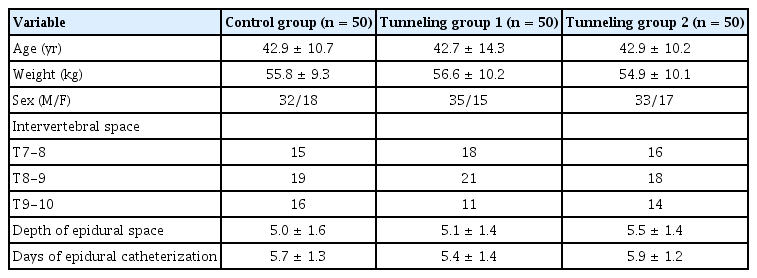
Patients undergoing major abdominal surgery were randomized into three groups of 50 patients each based on the method used to secure the epidural catheter. In the control group (CG), the epidural catheter was secured without tunneling. Tunneling groups 1 and 2 (TG1 and TG2) were defined as tunneling with and without a catheter loop, respectively. The primary outcome measure was the migration of the epidural catheter, while the secondary outcome measures were the adequacy of analgesia and signs of inflammation. All patients were followed up by the acute pain service team twice daily in the postoperative period until the epidural catheter was removed. The results were analyzed by the one-way analysis of variance (ANOVA), chi-square test, and Fisher’s exact test. P values <0.05 were considered significant.
Results
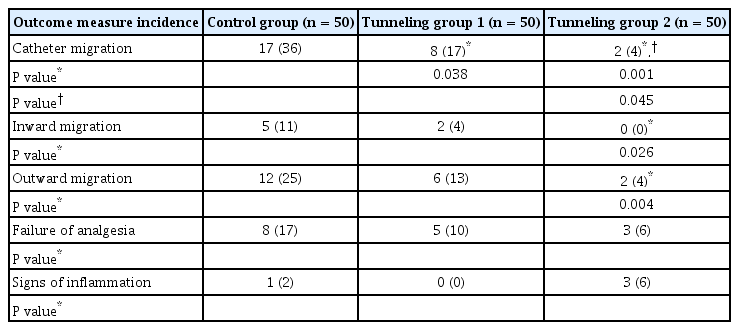
The three groups were similar with respect to patient characteristics. Catheter migration was significantly reduced in TG2 (two patients) compared to those in the other two groups, i.e., TG1 (eight patients) (P = 0.045) and CG (17 patients) (P = 0.001). No differences were found amongst the three groups in analgesia adequacy and catheter site inflammation (P > 0.05).
Conclusions
Catheter migration was significantly reduced by tunneling without a catheter loop in TG2 as compared to the other two groups. Therefore, we suggest routine use of tunneling without a catheter loop technique in anesthesia practice and look forward to future studies with larger sample sizes.
Introduction
Epidural analgesia is considered an ideal choice for the management of postoperative pain. It provides peri-operative analgesia superior to that provided by parenteral analgesics along with additional benefits including the reduced incidence of cardio-respiratory and gastrointestinal complications after major abdominal surgery [1,2]. Therefore, it is important to maximize the efficacy of postoperative epidural analgesia by minimizing the factors responsible for its failure in the postoperative period.
The displacement and migration of epidural catheters is a commonly reported equipment failure that results in inadequate epidural analgesia [3]. Technical issues in the form of premature epidural catheter withdrawal and catheter-bacterial filter assembly disconnection are responsible for epidural analgesia failure in up to 15% postoperative cases [3]. The standard methods of fixation cannot prevent migration in more than 50% of epidural catheters [4], which might lead to epidural analgesia failure if the catheter migrates outward or inward, giving rise to subdural, subarachnoid, or intravascular injection of drugs. Simple and cost-effective methods of catheter fixation with a low incidence of catheter migration are an appropriate answer to this problem.
The proposed epidural catheter fixation techniques include standard dressing, adhesive transparent dressing, tunneling, and epidural catheter clamp (Lockit clamp®, Smiths Medical, Czech Republic) [5–8]. Catheter migration is caused by patient movement [9]; spine flexion and extension [10]; spontaneous peeling of the adhesive dressing after getting wet due to perspiration, blood, discharges from the surgical incision, or skin movement during changes in patient positioning. Catheter migration secondary to skin movement cannot be avoided completely by fixing the catheter to the skin with the help of adhesive dressing; fixation techniques that minimize the traction on the catheter by skin rolling due to patient movement should be more effective in minimizing catheter migration.
This randomized control study compared the role of two epidural catheter tunneling techniques for the prevention of epidural catheter migration. The primary objective of this study was the assessment of epidural catheter migration.
Materials and Methods
The protocol for this randomized control study was approved by the institutional ethical committee (IEC code: IEC-07-IP-82) and registered in the Clinical Trials Registry-India (Registration number: CTRI/2016/11/007453). Written informed consent was obtained from all study participants. The clinical research was done following the ethical principles for medical research involving human subjects in accordance with the Helsinki Declaration 2013.
This study included adult patients aged 20–65 years with American Society of Anesthesiologists physical status I and II scheduled for major upper abdominal surgery under general anesthesia along with thoracic epidural analgesia. The surgical procedures included gastrectomy, colectomy, pancreaticoduodenectomy, exploratory laparotomy, and hepaticojejunostomy.
The exclusion criteria included patient refusal, uncontrolled systemic diseases, signs of local or systemic infection, coagulation disorders, and anatomical abnormalities.
Patients were recruited during the pre-anesthetic evaluation; each patient satisfying the inclusion criteria was randomly assigned to one of the three groups, with 50 patients in each group. Patient randomization was done by a project nurse not involved in the study with the help of a computer-generated table of random numbers. The random allocation sequence was sealed in an envelope; the anesthesia resident assigned to place the thoracic epidural catheter on the day of surgery opened these envelopes on the morning of surgery. The thoracic epidural catheter was placed in the T7 to T10 intervertebral spaces during the preoperative period under aseptic precautions. The epidural catheter (BD Perisafe®, Becton, Dickinson and Company, USA) was placed using a paramedian approach with loss of resistance technique; the epidural catheter with three lateral orifices was inserted with 5 cm of the catheter lying within the epidural space.
The epidural catheter was fixed along the patient’s back in the midline using sterile transparent adhesive film measuring 25 × 10 cm (Tegaderm®, 3M Healthcare, Germany); the catheter was fixed with the patient sitting upright or in a lateral position [9]. The transparent film permitted regular inspection of the epidural catheter; the dressing was changed if blood collection was found underneath the dressing during acute pain service rounds. The epidural catheters were secured as per the group allocation.
Control Group (CG): A loop of epidural catheter was formed at the catheter insertion site and fixed with transparent adhesive dressing tape.
Tunneling Group 1 (TG1: tunneling with a catheter loop): The epidural catheter was secured by tunneling. A subcutaneous tunnel of approximately 4 cm length was prepared by injecting 5 mL of 2% lignocaine with adrenaline along the paramedian plane, 1 cm lateral to the catheter insertion site; the catheter tunneling was facilitated by metal stylet of a 16-G intravenous cannula (Venflon®, Becton Dickinson Medical Pte Ltd., Singapore). The metal stylet was passed through the subcutaneous tunnel to exit 1 cm lateral to the catheter insertion site (Fig. 1). The distal end of the epidural catheter was passed through the stylet and pulled through the subcutaneous tunnel.
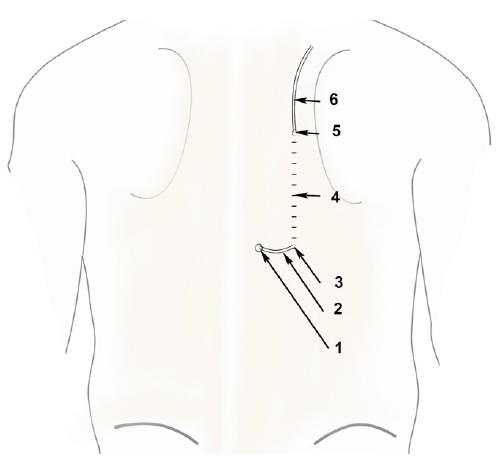
Tunneling group 1 (TG1).
1: Epidural catheter entry site, 2: Epidural catheter loop, 3: Epidural catheter entering the subcutaneous tunnel, 4: Subcutaneous tunnel, 5: Epidural catheter exiting the subcutaneous tunnel, 6: Epidural catheter.
Tunneling Group 2 (TG2: tunneling without a catheter loop): The epidural catheter was completely buried in the subcutaneous tunnel without any loop.
Catheter tunneling was performed in the following steps:
Step I: After epidural catheter placement in the epidural space, a Tuohy needle was withdrawn 2–3 cm with the catheter remaining within the needle to prevent catheter shearing during the preparation of the subcutaneous tunnel (Fig. 2A).

Steps of tunneling in tunneling group 2 (TG2). (A) The Tuohy needle is partially withdrawn and retained in the subcutaneous tissue with the epidural catheter. 1: Stab incision made alongside the Tuohy entry sites. (B) An intravenous (IV) cannula passed through the stab incision in a caudal direction along the subcutaneous tunnel and the distal end of the cannula punctured through the skin. 1: IV cannula entering the subcutaneous tunnel, 2: Tip of the IV cannula exiting the subcutaneous tunnel. (C) Epidural catheter passed through the subcutaneous tunnel of the intravenous catheter after removing the metal stylet and cutting the proximal hub of the cannula. 1: epidural catheter entering the IV catheter, 2: epidural catheter exiting the IV catheter.
Step II: A subcutaneous stab incision was made alongside the Tuohy entry site, and a 16-gauge intravenous (IV) cannula was passed through the stab incision (1 in Fig. 2B) subcutaneously for approximately 4 cm (2 in Fig. 2B).
Step III: The metal stylet of the IV cannula was removed and the proximal hub of the cannula was cut off, with the IV catheter with both its proximal and the distal ends jetting out of the skin (1 and 2 in Fig. 2C) acting as a subcutaneous tunnel. The Tuohy needle was withdrawn and the epidural catheter was threaded through the IV catheter tunnel. The IV catheter was gradually withdrawn, leaving the entire epidural catheter buried subcutaneously (Fig. 2C).
Outcome measures and assessment
The primary outcome measure was epidural catheter migration; the secondary outcome measures were analgesia adequacy and signs of inflammation.
All patients were followed up by the acute pain service resident twice daily in the postoperative period until the epidural catheter was removed. The anesthesiologist placing the epidural catheter initiated an acute pain service enrolment form containing patient demographic data, details of epidural catheter placement including the vertebral level of the catheter placement, depth of the epidural space, and catheter mark at the catheter insertion site in the CG and TG1 or at the tunnel exit site in the TG2. These catheter marks were also noted before catheter removal and the difference between two values was calculated as the catheter migration in individual patients. Inward migrations of 1 cm or more and outward migrations of 2 cm or more were considered significant [10]. Epidural analgesia was discontinued in cases of inadequate analgesia, with outward migrations exceeding 2 cm; inadequate analgesia was suggested by the requirement for alternative analgesia methods or replacement of the epidural catheter [11]. Each patient was also assessed daily for catheter migration, catheter dressing, analgesia adequacy, and catheter insertion site inflammation (defined as an area of erythema and induration >5 mm around the skin exit site and/or visible pus) [10].
Statistical analysis
The sample size calculation was based on the primary outcome measure, i.e., the incidence of catheter migration. Assuming that the epidural catheter tunneling would reduce the incidence of catheter migration from 30% in the control group to 5% in the tunneling group, a sample size of 43 patients was required in each group for the results to be significant (with α = 0.05 and power=80). To address dropouts, we enrolled 50 patients in each group. Demographic data were analyzed with one-way analysis of variance (ANOVA) and chi-square tests. The catheter migration incidence was analyzed with chi-square tests, while the incidence of analgesia adequacy and signs of inflammation were analyzed with Fisher’s exact tests. The method of analysis was decided prospectively and incorporated the intention-to-treat principle. IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., USA) was used to perform the statistical analyses. P < 0.05 was considered significant.
Results
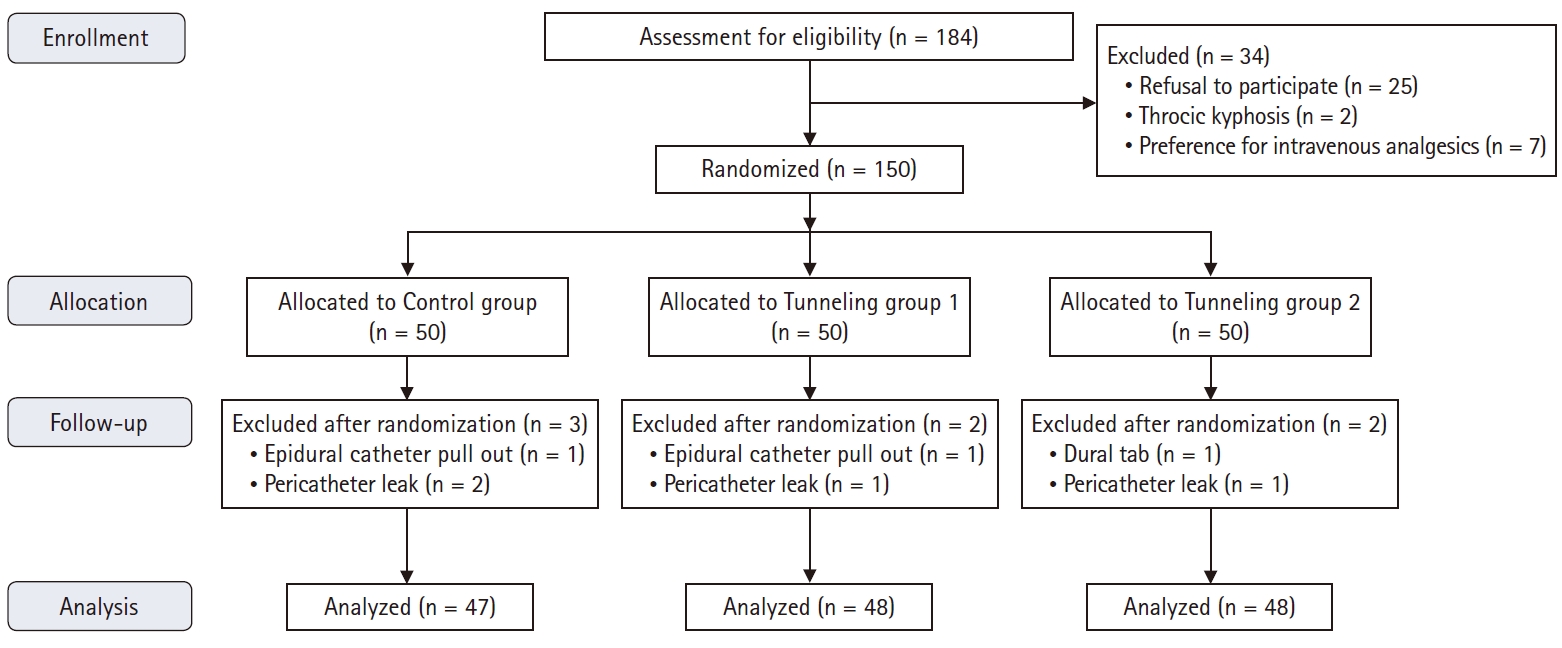
A total of 184 patients were assessed for participation in the study between March and August 2015; of these, 150 patients were included (Fig. 3). The study analysis could not be completed in seven patients as they were unable to continue epidural infusion (epidural catheter dislodged in two patients, one each during patient shifting and changing clothes, pericatheter leak in four patients, and dural tap in one patient during epidural catheter placement). These patients were included for analysis of patient characteristics but not for the analysis of catheter migration because the epidural catheter was withdrawn prematurely.
The three groups were similar with respect to patient characteristics, level of epidural catheter placement, length of epidural catheter placed inside the epidural space, and duration of epidural analgesia (Table 1).
Catheter migration was seen in all the groups, but was significantly lower in TG1 (eight patients, P = 0.038) and TG2 (two patients, P = 0.001) as compared to that in the CG (17 patients) (Table 2); there was also a significant reduction in catheter migration in TG2 as compared to that in TG1 (P = 0.045) (Table 2). The CG patients had a higher incidence of both inward and outward catheter migration as compared to those in TG2; none of the patients in TG2 had inward catheter migration. (Table 2).
A total of 16 patients reported analgesia failure (eight patients in the control group; five patients in the TG1 and three patients in the TG2); out of these sixteen patients, catheter migration was observed in eight patients (Table 2). Catheter site inflammation was observed in one patient in the CG and three patients in the TG2 (P = 0.617).
Discussion
The present study concluded that both tunneling techniques reduced epidural catheter migration. The tunneling without a catheter loop in TG2 appeared superior to other tunneling techniques used in TG1, as the incidence of epidural catheter migration in TG2 was one-fourth that in TG1.
The epidural catheter for postoperative epidural analgesia usually remains for three to five postoperative days; during this period, there was the possibility for catheter migration due to patient transfer, changes in patient position, patient ambulation, peeling of catheter dressing because of sweat or fluid discharge and friction between the patients’ back and the bed surface. These factors may result in traction on the epidural catheter by the skin and subcutaneous tissue due to skin rolling and movement.
The epidural catheter tunneling without a catheter loop in TG2 buries the epidural catheter subcutaneously so that there is only one site where the catheter traverses through the skin. In the traditional technique of epidural catheter tunneling in TG1, the epidural catheter traverses through the skin three times; hence, there is a possibility of greater skin traction being applied on the epidural catheter in TG1 as compared to that in TG2. This may have contributed to the lower incidence of epidural catheter migration in TG2 in the present study.
The incidence of epidural catheter migration in the present study was 4% in TG2 as compared to 17% in TG1 and 36% in the CG. We thought that a comparison of the results of the present study to those of previously published clinical trials would provide useful information. Thus, we applied the catheter migration criteria used in the present study to those of previously published studies and found an incidence of epidural catheter migration of 12–48% with transparent adhesive dressing [5,6] and 5–32% for various techniques used for epidural catheter fixation [6-10]. With epidural catheter tunneling, the incidence of inward catheter migration incidence was lower (4–12%) than that of outward migration (5–32%) [6,9,10]. Abukhudair et al. [12] reported no significant difference in the incidence of epidural catheter dislodgement between groups with or without tunneling; Sharma et al. [13] reported a significant increase in the incidence of side effects including erythema and bleeding with epidural catheter tunneling.
Analyses of catheter tunneling alone as a modality for epidural catheter fixation reported an incidence of epidural catheter migration associated with catheter tunneling of 12–32% [4,10,11] compared to 4% in the TG2 in the present study. Tripathi and Pandey [7] reported a 3% incidence of catheter dislodgement in the tunneling group but did not describe any details of outward catheter migration. Hence, the modified tunneling technique used in TG2 helped to reduce the incidence of epidural catheter migration.
In a multicenter registry analysis of 22,411 patients with continuous thoracic epidural analgesia, epidural catheter tunneling was associated with a lower risk of thoracic epidural catheter-related infections; however, the mechanism by which tunneling decreases infection is not clear [14]. The most common route of bacterial pathogen migration is along the cutaneous track of the epidural catheter [15]; epidural catheter tunneling allows better fixation, which reduces catheter movement underneath the skin, thus minimizing bacterial movement and colonization along the catheter [14]. The tunneling without a catheter loop in TG2 in the present study not only provided better catheter fixation but also shifted the catheter skin entry point at a distance from the site of entry into the epidural space. This may offer an additional barrier to bacterial infection.
The limitations of the present study are, first, the lack of blinding due to differences in the techniques used to secure the epidural catheter in the three groups. Second, the requirement for a small stab incision during epidural catheter tunneling in TG2, which resulted in catheter site inflammation in three patients in this group. Third, the study sample was inadequate to comment on analgesia failure or catheter insertion site inflammation. Finally, the directions differed between subcutaneous tunneling in TG1 and TG2; in addition to the presence or absence of a catheter loop, this might have also contributed to the reduced incidence of epidural catheter migration in TG2 as compared to that in TG1.
Catheter migration was significantly reduced by tunneling without a catheter loop in TG2 as compared to the other two groups. Therefore, we suggest routine use of tunneling without a catheter loop technique in anesthesia practice and look forward to future studies with larger sample sizes.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Sujeet Gautam (Conceptualization; Formal analysis; Methodology; Writing – original draft; Writing – review & editing)
Anil Agarwal (Data curation; Methodology; Project administration; Writing – review & editing)
Pravin Kumar Das (Data curation; Formal analysis; Investigation; Methodology; Supervision)
Sandeep Khuba (Investigation; Methodology; Resources; Software)
Sanjay Kumar (Investigation; Methodology; Project administration; Supervision)