Blood–brain barrier disruption caused by neonatal sevoflurane-induced depends on exposure time and is reversible in mice
Article information
Neonatal exposure to anesthetics, such as sevoflurane, induces neuronal apoptosis and results in learning deficits in rodents [1,2]. We showed that exposure to 2% sevoflurane for 6 h resulted in blood–brain barrier (BBB) disruption in the hippocampus of postnatal day 6 (POD6) mice [3]. There are no reports regarding whether neonatal sevoflurane exposure-induced BBB disruption are dependent on exposure time and whether they are reversible. The present study was performed to examine the length of time required for neonatal sevoflurane exposure to cause BBB disruption in mice. Furthermore, we investigated whether these changes were reversible.
The Animal Care and Use Committee of Tokyo Medical and Dental University approved the protocol in the current study (0170118A). C57BL/6NCr strain mice (male and female) at POD6 were used for the study. To reduce variability, the same number of pups from each litter were used for the same experiment.
Sevoflurane anesthesia was performed as previously described [2,3]. Briefly, POD6 mice were placed in a humid and warm chamber immediately after removal from the maternal cage. Sevoflurane (2%) was administered with 40% oxygen as the carrier gas for 6 h. Total gas flow was 1 L/min.
To study the effects of BBB disruption, POD6 mice were randomly divided into control or sevoflurane groups and exposed to carrier gas or sevoflurane, respectively (total number of pups used = 18). BBB disruption in the hippocampal CA1 region were examined using a transmission electron microscope (TEM) (H-7100; Hitachi, Japan) at 2, 4, and 6 h during sevoflurane exposure, and 24 and 48 h after sevoflurane exposure. Furthermore, we evaluated POD16 mice and 8-week-old mice that were exposed to carrier gas or 2% sevoflurane for 6 h.
Brain tissue were perfused with freshly prepared 4% paraformaldehyde and 2.5% glutaraldehyde. After dehydration, the hippocampal CA1 region was embedded in Epon 812, cut with a microtome, stained with uranyl acetate and lead citrate, and examined using TEM. We observed perivascular spaces because in our previous investigation [3] sevoflurane disrupted the perivascular spaces. We picked 15 capillaries in the hippocampal CA1 region for each animal sampled at each time point. The number of capillaries with destroyed perivascular space was counted and presented as a percentage of the number of capillaries with destroyed ultrastructure (%). Values are expressed as mean ± SD.
In the control group, the ultrastructure of capillaries in the CA1 region of the hippocampus was continuous, and the perivascular spaces were normal (Figs. 1A and 1F). However, the ultrastructural integrity was locally collapsed after 2 h of sevoflurane exposure (Figs. 1B and 1G). The perivascular spaces gradually became enlarged and collapsed depending on the sevoflurane exposure time (Figs. 1B–1D, 1G and 1H). To clarify these changes, Fig. 1J shows the quantification of BBB ultrastructural abnormalities (%). After 4 h of sevoflurane exposure, 64% of capillaries were destroyed and perivascular spaces were enlarged; 78% of capillaries were destroyed after 6 h of sevoflurane exposure.
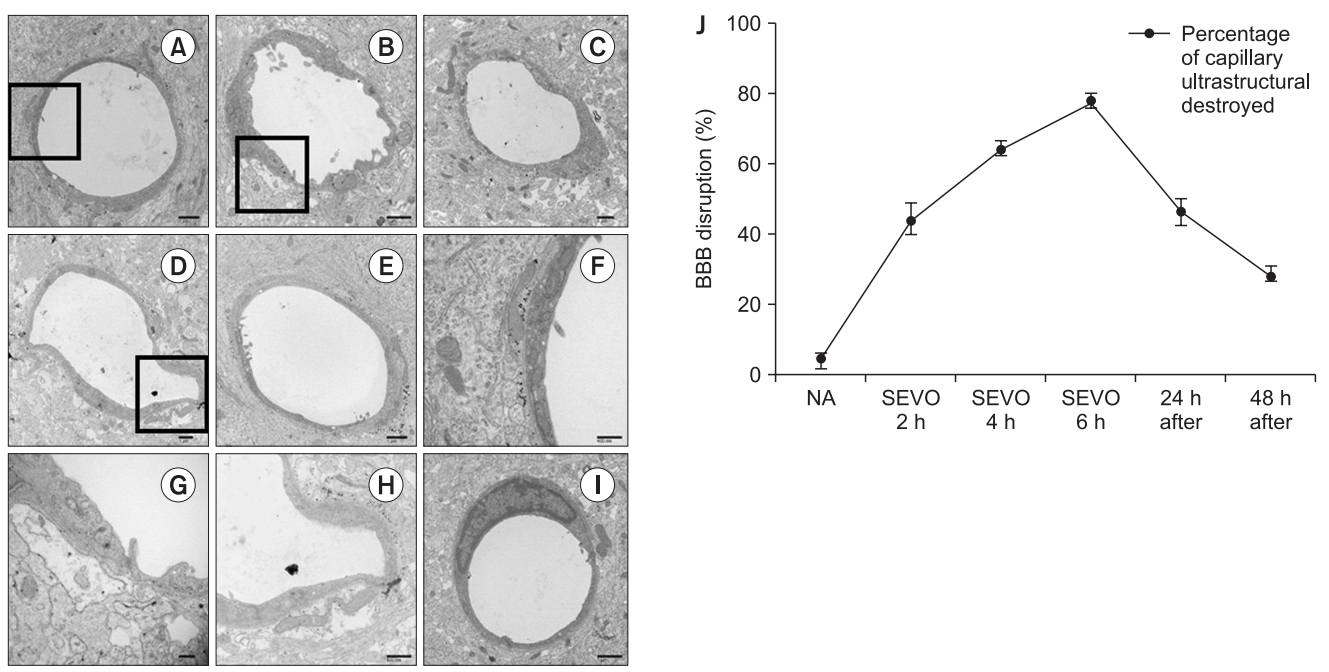
The ultrastructure of the capillaries (A, F) in the control group, (B, G) after sevoflurane exposure for 2 h, (C) after sevoflurane exposure for 4 h, (D, H) after sevoflurane exposure for 6 h, (E) after sevoflurane exposure for 24 h, (I) after sevoflurane exposure for 48 h, and (J) quantification of blood–brain barrier (BBB) disruption at each time point. The X axis shows sevoflurane exposure time and time after sevoflurane exposure for 6 h (24 h and 48 h after the end of anesthesia). In the control group, the capillaries were continuous and integrated, and the perivascular spaces were normal. However, the ultrastructural integrity of the BBB began to be disrupted after 2 h of sevoflurane exposure as perivascular spaces were enlarged. Sevoflurane exposure induced BBB opening in a duration-dependent manner. Additionally, the effect of sevoflurane exposure on the BBB opening was reversible as the enlarged perivascular spaces recovered gradually 24–48 h after sevoflurane exposure. The destroyed perivascular space was counted and presented as percentage of capillary ultrastructural destroyed (%). Values are presented as mean ± SD. n = 3 in each group. (F, G, and H) show higher magnifications of the insets square in (A, B, and D), respectively. Scale bars are shown on the right lower panels.
Next, we examined the reversibility of the BBB disruption. Twenty-four hours after removal of sevoflurane, 47% of capillaries were destroyed (Figs. 1E and 1J), and this dropped to 29% after 48 h (Figs. 1I and 1J).
The most vulnerable period for neonatal mice is during the first 2 postnatal weeks, which is also known as the brain growth spurt (BGS) period in rodents [4]. Therefore, we investigated whether the BBB disruption is dependent on the age at which sevoflurane exposure occurs. At 16 days after birth, 6 h of sevoflurane exposure had no influence on the BBB disruption (data not shown). Furthermore, at 8 weeks old, there was no destruction of the perivascular space in the hippocampal CA1 region (data not shown).
We showed that 2% sevoflurane caused BBB disruption in the hippocampus of mice at POD6. These abnormalities occurred only during the period of neuronal proliferation in the brain. Furthermore, we showed that these BBB disruption following neonatal 2% sevoflurane exposure were reversible.
The BBB was shown to open gradually with sevoflurane exposure time. Pups were returned to their dam after 6 h of anesthesia. At this time point, 78% of the BBB was disrupted, but this decreased to 47% after 24 h and to 29% after 48 h. Therefore, we confirmed that the BBB recovered after 2% sevoflurane exposure in POD6 mice. Meanwhile, even with exposure to 2% sevoflurane for 6 h, both POD16 and 8 week old mice showed no changes in the BBB. The neurotoxic effects appeared to occur during the period of brain development corresponding to synaptogenesis. This period corresponds to the BGS and is generally thought to occur between the third trimester of pregnancy and 3 years of age in humans, corresponding to the first 2 weeks postnatally in rodents [4]. Our results may explain the vulnerability of the developing brain to sevoflurane exposure.
This anesthetic protocol is often used in clinical practice in infants; however, a leaky BBB may lead to adverse outcomes under clinical conditions. It should be noted that there are differences between species, duration of anesthesia, and concentration of sevoflurane. Effects of anesthetics on rodents during BGS have been studied by exposing them to the anesthetic for long periods (4–6 h) [1–3]. A shorter period of exposure (30 min) to sevoflurane did not induce obvious long-term effects [5]. Moreover, morphological changes were observed using TEM in a manner dependent on the duration of anesthesia.
The results of the present study clearly showed that 2% sevoflurane exposure during the BGS caused BBB disruption in the hippocampus of mice at POD6. In addition, the BBB disruption after neonatal sevoflurane exposure in POD6 mice were reversible.
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Zhongliang Sun (Investigation)
Maiko Satomoto (Data curation; Funding acquisition; Writing – original draft; Writing–review & editing)
Yushi U Adachi (Writing–review & editing)
Koshi Makita (Supervision)
