Comparison of effect of electroacupuncture and nefopam for prevention of postanesthetic shivering in patients undergoing urologic operation under spinal anesthesia
Article information
Abstract
Background
Shivering during spinal anesthesia is a frequent complication and is induced by the core-to-peripheral redistribution of heat. Nefopam has minimal side effects and prevents shivering by reducing the shivering threshold. Electroacupuncture is known to prevent shivering by preserving the core body temperature. We compared the efficacies of electroacupuncture and nefopam for the prevention of shivering during spinal anesthesia.
Methods
Ninety patients scheduled for elective urological surgery under spinal anesthesia were enrolled in the study. Patients were randomly divided into the control group (Group C, n = 30), the electroacupuncture group (Group A, n = 30), and the nefopam group (Group N, n = 30). Groups C and A received 100 ml of isotonic saline intravenously for 30 minutes before spinal anesthesia, while Group N received nefopam (0.15 mg/kg) mixed in 100 ml of isotonic saline. Group A received 30 minutes of electroacupuncture before receiving anesthesia. Shivering scores, mean arterial pressure, heart rate, body temperature and side effects were recorded before, and at 5, 15, 30, and 60 minutes after spinal anesthesia.
Results
The incidence of postanesthetic shivering was significantly lower in Group N (10 of 30) and Group A (4 of 30) compared with that in Group C (18 of 30)(P < 0.017). Body temperature was higher in Group N and Group A than in Group C (P < 0.05). Hemodynamic parameters were not different among the groups.
Conclusions
By maintaining body temperature during spinal anesthesia, electroacupuncture is as effective as nefopam in preventing postanesthetic shivering.
Introduction
Shivering associated with regional anesthesia results from the core-to-peripheral redistribution of heat. Heat moves from the center of the body to the limbs because of vasodilation below the level of neuraxial block [1]. Shivering causes discomfort for the patients and may result in adverse effects, such as an increase in oxygen consumption, development of lactic acidosis, carbon dioxide generation, and an increase in left ventricular systolic work index [2].
Many pharmacological agents have been used to prevent postanesthetic shivering, but side effects including hypotension, bradycardia, sedation, and respiratory depression have been reported with their use [3456]. Nefopam is a non-narcotic, non-steroidal, centrally acting analgesic [7], that reduces the shivering-threshold, but does not affect the sweating or vasoconstriction thresholds. Therefore, nefopam has the advantage of minimizing heat loss because it induces a small increase in the core temperature. As a result, it is effective in the prevention and treatment of shivering. Unlike other drugs [89], nefopam maintains hemodynamic stability [1011], but does not induce respiratory depression or sedation.
A non-pharmacological treatment for the prevention of postanesthetic shivering is electroacupuncture (EA) [121314]. EA has been used in many anesthesia studies because, unlike manual acupuncture, it is easier to control and provides better replication of results, which is needed for the scientific approach [15]. EA has been reported to be effective in controlling postoperative nausea, vomiting [16], and pain [17]. Recent studies have reported that EA reduces postanesthetic shivering by preventing hypothermia [12131417]. To date there have been no studies comparing the efficiency of EA with pharmacological treatments for prevention of postanesthetic shivering. If the efficacy of EA is comparable to that of pharmacological treatments in preventing shivering under spinal anesthesia, EA may be an effective alternative method for prevention of postanesthetic shivering. In this study, we directly compared the effect of EA with nefopam on shivering, hypothermia, blood pressure, heart rate, and adverse effects during spinal anesthesia.
Materials and Methods
This study was a combination of open trial and double blind, prospective, randomized, controlled study. After the approval of the Institutional Review Board, informed consent was obtained from each patient. Ninety patients undergoing spinal anesthesia for elective urological surgery, from March 2016 to July 2016, at Dongguk University Gyeongju Hospital were enrolled in the study. Patients were 20–65 years of age with an American Society of Anesthesiologists physical status of I or II. Patients with uncontrolled hypertension, diabetes mellitus, severe obesity, fever (≥ 37.5℃), cardiopulmonary disease, hepatic or renal failure, and skin diseases were excluded. Patients underwent endoscopic surgery of the ureter, bladder and prostate, and surgery for incontinence and contraception.
Patients were randomly divided into 3 groups using the computerized random number generator. The groups were the control (Group C, n = 30), the EA (Group A, n = 30) and the nefopam (Group N, n = 30) groups. Before spinal anesthesia, all patients in Group C and Group A received 100 ml of isotonic saline solution intravenously over 30 minutes; patients in Group N received nefopam (0.15 mg/kg) diluted in 100 ml of isotonic saline over 30 minutes. To ensure blinding, all isotonic solutions were prepared by an anesthetic nurse not involved in the study. Patients and staff were unaware of the specific drugs used. The investigator recorded any side effects of nefopam such as intravenous injection pain, nausea, vomiting, dizziness, and palpitations.
Group A patients received EA at the PC 5 (Jianshi), PC 6 (Neiguan), ST 36 (Zusanli) and ST 37 (Shangjuxu) acupoints bilaterally, with 30 minutes of electrical stimulation before spinal anesthesia. One trained and licensed acupuncturists, with 20 years of experience, provided all EA for all EA patients in the operating rooms. Sterile, single-use, 30 mm × 0.25 mm stainless steel acupuncture needles were used for the study (Dongbang Acupuncture Inc., Boryeong, Korea). Needles were inserted vertically to a depth not exceeding 1.3 cm. As the appropriate depth was reached, the "Deqi" sensation was evoked manually. The handle of each needle was connected with crocodile clips to the electro stimulator (PG 306, Suzuki Ltd, Tokyo, Japan, Fig. 1). The frequency of stimulation was 3 Hz and the intensity was set at an appropriate grade for patients to feel strongly stimulated, but not to cause pain. The investigator recorded side effects of EA, such as acupuncture pain, itching, skin rash, and burns caused by the acupuncture needles.

(A) The acupuncture points of forearm. The acupoints PC 5 (Jianshi) and PC 6 (Neiguan) are located on 3 cun (the measurement of 1 body inch used locate acupuncture points, 1 cun = the width of the thumb) and 2 cun above the wrist crease between palmaris longus and flexor carpi radialis tendons, on the line connecting middle of the transverse crease of the wrist and cubital fossa. (B) Electroacupuncture at the PC 5 and PC 6. (C) The acupoints of lower leg. The ST 36 (Zusanli) is located on 3 cun below ST 35 (Dubi, below the patella in a depression lateral to the patella ligament identified with the knee flexed) 1 finger width lateral from the anterior crest of the tibia, in the tibialis anterior muscle. ST 37 (Shangjuxu) lies at 3 cun below ST 36. (D) Electroacupuncture at the ST 36 and ST 37.
Spinal anesthesia was performed with the patient in the left lateral decubitus position. A 25-gauge Quincke spinal needle was inserted in the L3–4 interspace and 10–14 mg of 0.5% hyperbaric bupivacaine was injected intrathecally. The anesthesiologist used alcohol swabs to confirm the sensory block level at 5 minute intervals after spinal anesthesia until the beginning of surgery, and recorded the level of sensory block, the total dose of bupivacaine, and the total duration of surgery.
The primary outcome measures of this study were tympanic membrane temperature and the degree of postanaesthetic shivering. The core body temperature was measured as the tympanic membrane temperature with an infrared tympanic thermometer (ThermoScan IRT 4020, Braun, Kronberg, Germany) [1]. Temperature measurements were done before administering anesthesia (baseline), and at 5, 15, 30, and 60 minutes after spinal anesthesia.
The degree of postanesthetic shivering was evaluated immediately after spinal anesthesia, and 5, 15, 30, and 60 minutes after spinal anesthesia. Shivering was evaluated using a 5-point scale. The scale used was; 0 = no shivering; 1 = observation of one or more of the following: piloerection, peripheral vasoconstriction, peripheral cyanosis without other causes, and/or no visible muscular activity; 2 = visible muscular activity confined to 1 muscle group; 3 = visible muscular activity in more than 1 muscle group; 4 = gross muscular activity involving the entire body. If more than 2 points of shivering occurred, 25 mg of meperidine was administered intravenously. Throughout anesthesia and surgery the operating room temperature was maintained at 22℃ and no active warming techniques were used for patients.
The secondary outcomes, including mean arterial pressures, heart rates, and adverse effects (hypotension, bradycardia, nausea, and vomiting) were measured at 5, 15, 30, and 60 minutes after spinal anesthesia. For this study, hypotension was defined as a decrease in mean arterial pressure below 60 mmHg or a greater than 20% decreases from baseline. When hypotension occurred, ephedrine 5 mg was administered intravenously. Bradycardia was defined a heart rate below 50 beats/min or a decrease in heart rate of greater than 20% of the baseline. Atropine 0.5 mg was administered intravenously for bradycardia.
The IBM SPSS 20.0 (IBM Corp., Armonk, NY, USA) program was used to analyze the statistical data. To compare continuous data measured over time (mean arterial pressure, heart rate, and tympanic membrane temperature) among groups we used repeated measures ANOVA with Fisher's least significant difference for post-hoc analysis. Continuous variables were analyzed using ANOVA test and categorical variables were analyzed using chi-square test or Fisher's exact test. Sensory block levels and the degree of shivering were analyzed using Kruskal-Wallis test with post-hoc analysis using Mann-Whitney U test for each pair of groups. A Bonferroni's corrected P value of 0.05/3 (P < 0.017) was considered statistically significant. Data are presented as mean ± SD, median (Q1–Q3), or absolute number, with percent of group shown as (%) after the number. P value < 0.05 was considered statistically significant.
Results
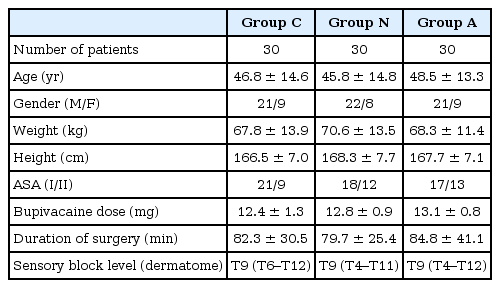
Patients' demographic characteristics, duration of surgery, bupivacaine doses, and sensory levels were not difference among the groups (Table 1).
The tympanic membrane temperatures decreased over time, and no significant differences among the groups were noted at the 5 or 15 minute time point. The tympanic membrane temperature of Group C was significantly lower at 30 and 60 minutes compared with those of Group N or Group A (P < 0.05). At 30 and 60 minutes there was no significant difference in the tympanic membrane temperatures between group N and A (P > 0.05, Fig. 2).
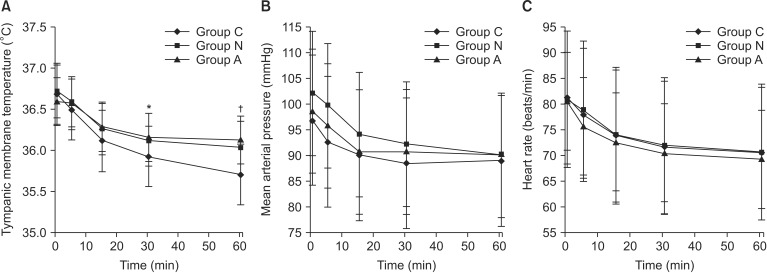
Changes of tympanic membrane temperature, mean arterial pressure, and heart rate after spinal anesthesia. (A) tympanic membrane temperature, (B) mean arterial pressure, and (C) heart rate. Group C: control group, Group N: nefopam group, Group A: electroacupuncture group. Data are mean ± SD. When compare to that of other groups, the tympanic membrane temperature of Group C was lower at both 30 minutes (*P < 0.05) and 60 minutes (†P < 0.05). No differences in mean arterial pressure or heart rate were detected among the groups.
The shivering score was significantly different among groups (P < 0.001). Group C had higher shivering scores than either Group N or Group A (1 [0–4], 0 [0–4], 0 [0–3], respectively). There was no significant difference in shivering scores between Group N and Group A. The number of patients without shivering (shivering score 0) was significantly higher in Group N (n = 27, 90%) and Group A (n = 26, 87%), when compared with those in Group C (n = 12, 40%, P < 0.017), but no differences were noted between Group N and Group A (P > 0.017). The number of patients with mild shivering (shivering score 1) was significantly lower in Group N (n = 2, 7%) and Group A (n = 3, 10%) when compared with that in Group C (n = 10, 33%, P < 0.017), and no differences were detected between Group N and Group A (P > 0.017). However, moderate to very severe shivering scores (shivering scores 2–4) were not significantly different among the groups (P > 0.017). The total number of patients that required treatment for shivering (shivering score ≥ 2) was significantly greater in Group C (n = 8, 27%) than Group N (n = 1, 3%) or Group A (n = 1, 3%, P < 0.017, Table 2). All patients who experienced grade 2 or higher shivering scores received meperidine and subsequently their condition improved.
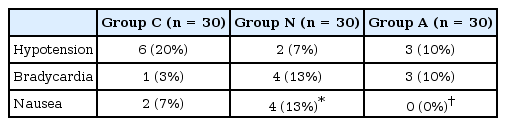
The mean arterial pressures and heart rates decreased over time, but no significant differences were observed among the groups (P > 0.05, Fig. 2). The incidence of hypotension and bradycardia did not significantly differ among the groups. These side effects were successfully controlled by the administration of ephedrine or atropine. The incidence of nausea in Group A was significantly lower than in Group N (P < 0.05), but did not differ between Group C and Group A, and Group C and Group N (P > 0.05, Table 3).
Patients in Group N complained of injection pain (n = 5, 17%) and nausea (n = 4, 13%), but other adverse effects such as vomiting, dizziness, palpitations were not observed. Patients in Group A complained about acupuncture pain (n = 6, 20%) and skin rash (n = 3, 10%), but itching and burns caused by the acupuncture needles were not reported.
Discussion
Shivering is a common complication associated with regional anesthesia and may occur in 55% of patients receiving neuraxial anesthesia [18]. Many drugs, including clonidine, dexmedetomidine, tramadol, alfentanyl, and meperidine, have been used to reduce the uncomfortable effects of shivering and prevent shivering related to anesthesia. Although these drugs reduce shivering, they have side effects such as hypotension, bradycardia, sedation, nausea, vomiting, dry mouth, itching, and respiratory depression [3456].
Nefopam is a non-narcotic, non-steroidal, centrally acting analgesic belonging to the benzoxazocine chemical class. Although the pharmacological mechanism remains unclear, it has been reported to inhibit reuptake of serotonin, norepinephrine, and dopamine [71920], and is known to act as a non-competitive N-methyl-D-aspartate-receptor antagonist [2122]. When nefopam is administered intravenously, a single 20 mg dose should be infused slowly over 15 to 20 minutes to prevent adverse effects such as nausea, cold sweating, dizziness, tachycardia, drowsiness, and injection pain [7]. While the optimum dose of nefopam to prevent shivering has not been determined, Kim et al. [6] demonstrated that nefopam at a dose of 0.15 mg/kg provided anti-shivering effects equivalent to that of meperidine. Based on these studies, we chose a dose of 0.15 mg/kg of nefopam administered over 30 minutes to minimize adverse effects and to match the time with the EA group.
Acupuncture and related techniques have been used to prevent postoperative pain, nausea, and vomiting, and to reduce preoperative anxiety, postoperative shivering and emergence delirium [23]. EA is a type of electrotherapy that involves passing an electrical current between pairs of acupuncture needles that are attached to a device that generates an electrical pulse [15]. The mechanism of EA that prevent shivering has not been elucidated. Stener-Victorin et al. [24] proposed that the low frequency (below 10 Hz) EA, at acupoints of lower extremities, attenuates sympathetic nerve activity, which may mediate muscle shivering. EA also leads to peripheral vasoconstriction and interrupts the core-to-peripheral redistribution of heat that results from spinal anesthesia [12]. Furthermore, several experimental studies describe the effects of acupuncture for the prevention and treatment of shivering. Yeh et al. [12] stated that when EA is performed at low frequency (3 Hz) on ST 36 and ST 37, the core temperature is elevated and the redistribution of heat is inhibited, resulting in a reduced incidence of postanesthetic shivering. Lin et al. [13] reported that when ST 36 was stimulated, oral temperature was increased, while cutaneous temperature of the limbs was reduced. Shoar et al. [14] reported that preoperative EA on Hegu (LI 4) and PC 6 resulted in less frequent postoperative shivering. In addition, Chen et al. [17] reported that when EA was performed on LI 4 and PC 6, postoperative pain, postoperative nausea and vomiting, and shivering were significantly reduced. Based on these studies, we hypothesized that the low frequency EA at PC 6 (Neiguan) and ST 36 (Zusanli) may prevent postanesthetic shivering by inducing peripheral vasoconstriction and maintaining core temperature. We selected the additional acupoints, PC 5 (Jianshi) and ST 37 (Shangjuxu) that are adjacent to PC 6 and ST 36, respectively. In oriental medicine, penetrating adjacent acupuncture points in 2 or 3 places, enhances the therapeutic effect of the existing acupoint [14].
In our study, core temperature was significantly higher in patients receiving nefopam or EA when compared to that in the control patients. There were no differences in factors influencing body temperature, such as type of surgery, operation time, and operation room temperature. Demographic characteristic were not different among the groups. Because nefopam and EA increase the core temperature by different mechanisms [812], these different results might be attributable to the different mechanisms of action of nefopam and EA.
Our results are in agreement with other studies that show that the incidence of shivering was low in patients in the nefopam and EA groups when compared with those in the control group. In our study, the number of patients with moderate to very severe shivering (grade 2–4) was not significantly different among the groups. However, the number of patients who needed treatment for shivering was greater than in the control group. These data suggest that both nefopam and EA can effectively prevent of shivering after spinal anesthesia.
Nefopam, by maintaining hemodynamic values like blood pressure and heart rate, is superior to other anti-shivering agents in preventing shivering [611]. Prior to our study, there were no reports in human patients that EA was also an effective treatment for maintaining hemodynamic stability. In animal studies, EA at the PC 5 and PC 6 influences vascular pressure responses and the cardiovascular sympathetic nervous system [2526]. In particular, EA at the PC 6 point augments sympathetic tone and improves cardiovascular function. Thus, EA increases cardiovascular parameters such as stroke volume and cardiac output and decreases hemorrhagic hypotension [2728]. We hypothesized that the mean arterial pressures and heart rates in patients in the nefopam and EA groups would be higher than control group. However, our study showed, no significant differences in hemodynamic values among the groups.
Common side effects of nefopam are nausea, palpitations, tachycardia, and injection pain. These side effects may limit the routine use of nefopam for preventing shivering, and slow infusion of drug is recommended to minimize side effects. In our study, sweating or tachycardia was not observed; however, the incidence of nausea in the nefopam group was statistically higher than the EA group. This difference between the two groups was due to the adverse effect of nefopam and the antiemetic effect of EA [16]. Although nefopam was administered slowly, injection pain was observed; however, the pain was not severe and resolved spontaneously within 30 minutes.
There are several disadvantages of the use of EA. First, because of continuous electrical stimulation, EA requires longer duration of therapy than other methods. Patients may experience discomfort, pain, and skin disorders, such as a redness, swelling, and tenderness at the acupuncture sites [15]. In our study, acupuncture pain and skin rash occurred in Group A patients, but they did not last more than 10 minutes and the patients did not require treatment. Second, an experienced acupuncturist performed all the EA treatments to induce precise clinical effects; not all medical facilities have access to skilled and experienced acupuncturists, thus this treatment may not be available in all surgical settings. In such cases, transcutaneous-electrical-acupoint stimulation may provide an alternative therapy, because it is not invasive and can be applied by medical personnel with minimal training [17].
Our study is the first controlled investigation comparing EA directly to nefopam and non-treatment for the control of shivering. The results suggest that EA can be an alternative method to control postanesthetic shivering after spinal anesthesia. Since EA has no adverse effects like nausea and injection site pain with EA, when compared to nefopam, it may improve the patients' perioperative comfort.
There are several limitations in our study. Primarily, in contrast with other groups, the patients in the EA group were not blinded to the treatment before spinal anesthesia. The efficacy of EA was previously explained, hence subjective outcomes (such as shivering) but not objective outcomes (such as core temperature and hemodynamic values) might have been influenced by the patients expectations. Another limitation is that we did not check peripheral skin temperature. While nefopam does not affect vasoconstriction thresholds it does reduce shivering threshold [89], and, EA directly induces peripheral vasoconstriction [1213]. In our study, the core temperatures in the nefopam and EA groups were not significantly different. Therefore, measuring the skin temperature might have provided information about the differences in the anti-shivering mechanisms of nefopam and EA. Finally, the duration of our study was short. Patients requiring other surgeries, like orthopedic or plastic surgery, were not able to received EA because of their leg wounds, and in obstetric patients, EA is avoided because it can cause unexpected results [15]. We selected patients undergoing urological surgery for this study because these surgeries are of relatively short duration. With longer duration operations, the risk of hypothermia and shivering increases. Alfonsi et al. [8] showed that nefopam delays the onset of shivering for two hours, while the effective duration of EA was less than two hours [15]. If we increased the duration of study to over one hour, the resulting changes of core temperature or frequencies of shivering might have been different between the nefopam and EA groups.
In conclusion, EA on specific acupoints is as effective as nefopam for the prevention of postanesthetic shivering. EA prevents shivering by maintaining the body temperature during spinal anesthesia. EA can be a viable alternative for prevention of postanesthetic shivering, and overcoming technical difficulties, extending the duration of stimulation, and minimizing adverse effects can lead to increased use or EA to manage postoperative shivering.